Application of three-dimensional printed biomodels in endoscopic spinal surgery
Highlight box
Key findings
• Three-dimensional printing (3DP) improved surgical planning and operational learning curve for endoscopic spinal surgery.
What is known and what is new?
• 3DP has known benefits to planning, operations and training in other surgical fields.
• 3DP has similar benefits in endoscopic spinal surgery.
What is the implication, and what should change now?
• Evidence to support increased use of 3DP in planning and surgical training for spinal surgery.
Introduction
Background
Three-dimensional printing (3DP) is an additive manufacturing process that fabricates complex and unique objects in layers and at lower costs than existing manufacturing methods (1). Producing one-off objects enables patient-specific materials, such as prosthetics, medical implants, operative templates, and anatomical models which further individualise surgeries (1,2). The most widespread applications of 3DP are patient anatomical models known as biomodels, which assist surgeons in understanding patient anatomy (3). The field of spinal surgery stands to benefit from biomodels as operations require understandings of complex anatomy and increasingly utilises minimally invasive techniques with endoscopic approaches (3). Endoscopic spinal surgery presents unique operational challenges due to complex anatomy with small exposure corridors, difficult visualisation, minute working spaces, low tolerances for error and a difficult learning curve (4). Therefore, there are multiple challenges in endoscopic spinal surgery which may be improved with biomodels.
One advantage of biomodels is that they represent anatomy in a more easily understood form, which accelerates surgical planning, intraoperative decision-making, and surgical education. Routinely, surgical planning is based off two-dimensional computerised tomography (CT) and magnetic resonance imaging (MRI) scans which are mentally constructed into three-dimensions. A biomodel physically represents the anatomy without requiring mental visualisation, circumventing a challenging and complex task (2). In addition, handling a biomodel is reported to shorten planning time through providing tactile feedback, improving spatial awareness and by enabling simulated procedures with surgical equipment (5,6). Additionally, surgeons require understandings of patient anatomy to plan the surgical approach and trajectory of approach. For spinal surgery, multiple approaches exist with varying advantages and disadvantages, and a biomodel may help determine the best choice for each patient (7). For example, surgeons trained in interbody techniques for lumbar interbody fusion tend to favour one technique, but biomodels may highlight anatomical indications for another approach (7). In a literature review, the most documented benefit of biomodels across all fields of surgery was during preoperational planning, such as aiding the selection of the most appropriate equipment and helping surgeons anticipate difficulties in the surgery (8). Of the limited spinal surgery studies, Izatt et al. reporting vertebral anatomical details were more visible on the biomodel in 65% of cases than two-dimensional imaging scans and exclusively visible on the biomodel in 11% of cases (9).
Additionally, intraoperative benefits correlated to biomodel use within surgical planning across multiple studies, including more efficient, shorter operations with reduced trauma, bleeding, and overall risk to the patient (1,10,11). A systematic review noted biomodels correlated to 15–20% reductions in operation time in multiple procedures and studies (1). Furthermore, several case series reported that surgeons favoured biomodels over conventional imaging and noted biomodels reduced the likelihood of altering surgical plans during tumour resection operations (12,13). The extent of benefit from biomodels are hypothesised to increase with the anatomical complexity of the surgery, which may explain the early uptake of biomodels in orthopaedic, oral and maxillofacial surgery (11). In a study of complex spinal surgeries, biomodels reduced the operating time by an average of 22%, slightly more than those reported in other systematic reviews. Another study of Lenke type 1 adolescent idiopathic scoliosis correction found significantly reduced misplacement of pedicle screws compared to cases without a biomodel (9,14). The same benefits may apply to endoscopic techniques, with reduced postoperative complications in 13 patients undergoing thoracic ligamentum flavum ossification repair (15).
However, benefits are not always present when biomodels are used in operational planning. A review of 89 studies noted 2.24% reported increases to operation times (n=2), 41.6% reported no changes (n=37) and 53.9% mentioned reduced operating room time (n=48) (16). However, the reported benefits may not translate practically because of positive bias in research publication. Furthermore, biomodels are reported to be most useful in patients with complex anatomy (17). In these cases, the disadvantages of added expense and time delay for printing the model are less significant (17). Additionally, one study reported reduced operation time and blood loss without changes in patient outcomes and complication rates in a small sample size (n=37) (18). As a range of studies report no changes in outcomes or varying benefit depending on case complexity, further research is required on the practical impacts of biomodels (18,19).
Biomodels may also benefit surgical education and the learning process for operations. All operations involve a learning curve, and it is critical to support trainees learning new surgical procedures as the early learning phase exposes patients to additional risk (20,21). Biomodels of normal and pathological anatomy may improve trainees’ knowledge of underlying structures and abnormalities. Trainees may then practise on the biomodel to further improve understandings of pathological anatomy and improve their operational competence. It is well-documented that improved learning speed is associated with increased patient safety, reduced complications, reduced open procedure conversions, shorter operations, and shorter hospital stays (21). In one study, biomodels assisted trainee surgeon education in 89% of cases and enabled simulated endoscopic spinal surgery procedures (9). This reflects reports of improved resident performance in surgical simulations and medical student performance in comprehension tests when provided biomodels (18,19). However, 3DP is currently unable to imitate the texture of tissue for procedural training (18,19).
Rationale and knowledge gap
In addition to planning and operational benefits, there are reported improvements to surgical education. This is particularly valuable in neurosurgery due to the limited focus on endoscopy training in Australia. Contrastingly, other specialties such as orthopaedics mandate training in knee and shoulder arthroscopy. However, the literature consists of minimal, low-quality studies without clear consensus regarding the extent to which biomodels may improve spinal surgery education, planning and intraoperatively.
Objective
To evaluate the preoperative, intraoperative, and learning benefits when 3DP biomodels are provided for surgical cases. We present this article in accordance with the STROBE reporting checklist (22) (available at https://jss.amegroups.com/article/view/10.21037/jss-23-103/rc).
Methods
Materials
This was a prospective study of biomodels produced for a series of elective vertebral endoscopic surgeries (n=33), including combinations of microdiscectomy (n=27), foraminotomy (n=7), and laminectomy (n=3). These surgeries were conducted at vertebral levels ranging from L2/3 to L5/S1. Patient characteristics are represented in Table 1, and patients were selected with no eligibility criteria if the operation was performed between February 2022 and February 2023 at Prince of Wales Private Hospital, Sydney, Australia. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the South Eastern Sydney Local Health District Human Rights Ethics Committee (HREC) (HREC: 2021/ETH11998) and all patients (n=33) provided consent with no patient follow-up conducted.
Table 1
| Case characteristics | Number |
|---|---|
| Surgery included vertebral level† | |
| L2/3 | 3 |
| L3/4 | 9 |
| L4/5 | 19 |
| L5/S1 | 4 |
| Procedure type† | |
| Microdiscectomy | 27 |
| Foraminotomy | 7 |
| Laminectomy | 3 |
| Total | 33 |
†, some operations include multiple vertebral levels and procedures
The biomodel was present as a tool to understand patient anatomy and to conduct simulated surgeries. At the preoperative planning stage, both the biomodel and imaging scans were available to determine the characteristics of the surgery, such as the approach angle, incision location and the angle of scope insertion. Each model was sterilised for use as an intraoperative reference. The Elliquence endoscopic system (Elliquence, Baldwin, NY, USA) was used to access the foramen.
Biomodel production
Patient CT scans were acquired for model generation and was limited to the patient’s pathology to minimise radiation exposure. Following this, the CT digital imaging and communications in medicine (DICOM) images were reconstructed into digital three-dimensional models using 3 Matic (vs. 14.0) software (Materialise, Leuven, Belgium). This digital model was then 3DP with stereolithography from Form 2 clear resin (Formlabs, Somerville, MA, USA) at a local biomodel supplier (Specifica Pty Ltd., Caringbah, Australia). One vertebral model typically required 4 hours to process for model production.
Data collection
After completing all procedures, the surgeon completed a structured, qualitative post-surgical assessment questionnaire regarding how the biomodel impacted preoperative planning, intraoperative decision-making and learning the operation. To reduce recall bias and selection bias, each questionnaire was completed immediately after the operation and all patients were invited to the study without selection. At the conclusion of the study, an unstructured interview recorded the surgeon’s overall opinion on the usefulness and effectiveness of the biomodel.
Statistical analysis
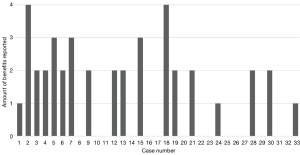
The data from the questionnaire provided the number of cases with preoperative (n=18), intraoperative benefit (n=20) or benefit to learning the operation (n=3), with some cases reporting multiple categories of benefit (Figure 1). These totals were then divided by the total number of cases (n=33) to provide the percentages of preoperative (54.5%), intraoperative (60.6%) and operational learning (9.09%).
Results
There was a reported preoperative benefit in 54.5% of cases (n=18), with some operations having multiple reported benefits. The most frequent benefit was an improvement to foraminal drill understanding (n=11). Other preoperative benefits were improving anatomy comprehension (n=9) and determining the angle of scope introduction (n=9), while an improved understanding of disc herniation position was the least reported benefit (n=7) (Table 2). Preoperative benefits were more frequent in the first third of the cases (n=8) than in the middle (n=6) and last thirds (n=4) (Table 3). Additionally, more aspects of preoperative planning were improved earlier in the cases (Figure 1).
Table 2
| Benefit on operation | Number (N=33) |
|---|---|
| Assisted with preoperative planning in any way† | 18 |
| Foraminal drill understanding | 11 |
| Understanding patient anatomy | 9 |
| Angle of scope introduction | 9 |
| Understanding disc herniation position | 7 |
| Assisted with intraoperative decision-making | 20 |
| Assisted with the learning curve | 3 |
†, some operations reported more than one form of preoperative benefit.
Table 3
| Case number | Preoperative benefits† | Intraoperative benefits† | Operation learning curve benefit† |
|---|---|---|---|
| 1–11 (n=11) | 8 | 10 | 3 |
| 12–22 (n=11) | 6 | 4 | 0 |
| 23–33 (n=11) | 4 | 7 | 0 |
†, some cases reported no benefit or more than one type of benefit.
Intraoperatively, biomodels assisted decision-making in 60.6% of cases (n=20). These improvements result from the improved planning process and the intraoperative reference biomodel. For example, one case was assisted with the biomodel during bone exposure difficulties due to large, sequestered bone fragments. Overall, all forms of benefits were more frequently reported in the first third of cases.
With regards to surgical learning, the surgeon stated that biomodels accelerated the early learning curve of the operation three times. However, these improvements only occur within the first five operations in the series or 60% for early cases. Additionally, the surgeon commented that the biomodels were more frequently used early in the cases and were more beneficial early as well. Overall, 75.8% of cases reported some benefit (n=25) and a minority of cases (n=8) recorded no benefit.
Discussion
Key findings
This study reported preoperative and intraoperative benefits in most cases. Similar studies of 3DP in endoscopic spinal surgery reported more visible anatomy on the biomodel in 76% of cases (9). This figure is comparable to the finding that the surgical planning of most cases in this study was improved with the biomodel (54.5%). Other systematic reviews also note the preoperative benefits reported in this study regarding understanding foraminal drill specifics, angle of scope introduction, and understanding of disc herniation position (23). Interestingly, Table 3 highlights more benefits were reported early in the series. This trend is consistent with the surgeon’s comments that the models were more utilised and impactful at the beginning of the cases.
Strengths and limitations
This study is limited to documenting pre-operative and intraoperative benefits as post-operative outcomes for patients was not investigated. Furthermore, one surgeon was involved in this study, potentially introducing observer bias.
Comparison with similar researches
The incidence of intraoperative benefits (60.6%) does not directly correspond to metrics of improved operation time used in other studies. However, spinal surgery studies report a similarly high incidence of operational time reduction (24). For other surgical specialities, benefits of reduced operation length and intraoperative guidance were reported in 32.9% and 24.1% of papers within a literature review (8).
Explanations of findings
Interestingly, biomodels were more beneficial early in the series, preoperatively and intraoperatively (Table 2 and Figure 1). This trend is corroborated by the surgeon’s comment that models were more useful early on, signifying increased early benefit from biomodels in the cases. Furthermore, this trend applied to improving the learning of the operation as all benefits occurred in the first five cases. The main components of a learning curve are decision-making and technical factors, with endoscopic surgeries known to have a particularly difficult learning curve (20). This reported assistance to the early learning curve agrees with previous studies reporting biomodels improved the performance of surgical trainees learning pedicle screw instrumentation technique (18,19).
Implications and actions needed
Overall, there is a significant incidence of improvements to the operation reported preoperatively (54.5%) and intraoperatively (60.6%). In addition, biomodels assisted for much of the early learning curve for a new technique during the first five cases (60%) but not later in the series. This supports increased use of 3DP in spinal surgery but requires further investigation especially regarding the value of intraoperative improvements.
Conclusions
3DP is a promising technology for spinal surgery with documented advantages across surgical specialties. Printed biomodels improved understandings of patient-specific anatomy and reported benefits in this study of endoscopic spinal surgeries. There was improved preoperative planning and intraoperative benefits in most cases (54.5% and 60.6%, respectively) and improved early surgical learning (60%) for the first five procedures. Additionally, this study found the biomodel was more beneficial in the earlier cases, and improvements to learning the operation were only reported in the first five procedures. This especially apparent for improvements to the learning curve, with this trend reflected in the comments of the spinal surgeon regarding the study. The incidences and types of benefit are similar to the literature, such as biomodels informing optimal scope introduction angle. This study was limited by reporting qualitative comments from a single surgeon. Further high-quality research with standardised and more comprehensive reporting of benefits and disadvantages is required. In addition, investigation of the impacts on patient outcomes may provide sufficient evidence to support biomodels as a resource routinely utilised for endoscopic spinal surgery. Furthermore, biomodels and virtual holographic technologies may be used to improve the early phase of surgical training for complex spinal surgeries. While this technology is refined and increasingly adopted, further studies are required to comprehensively assess the benefits and disadvantages of biomodels and determine its viability as a widespread resource.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-103/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-103/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-103/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-103/coif). R.J.M. serves as the Editor-in-Chief of Journal of Spine Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee (HREC) (HREC: 2021/ETH11998) and all patients provided consent with no patient follow-up conducted.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilcox B, Mobbs RJ, Wu AM, et al. Systematic review of 3D printing in spinal surgery: the current state of play. J Spine Surg 2017;3:433-43. [Crossref] [PubMed]
- Tejo-Otero A, Buj-Corral I, Fenollosa-Artés F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann Biomed Eng 2020;48:536-55. [Crossref] [PubMed]
- Hsu MR, Haleem MS, Hsu W. 3D Printing Applications in Minimally Invasive Spine Surgery. Minim Invasive Surg 2018;2018:4760769. [Crossref] [PubMed]
- Kim HS, Wu PH, Jang IT. Maximal Benefit Zone of Endoscopic Spine Surgery in the Cervical and Thoracic Spine: Rationale of Endoscopic Spine Surgery in the Cervical-thoracic Region. J Minim Invasive Spine Surg Tech 2023;8:1-4.
- Kumar N, Alathur Ramakrishnan S, Lopez KG, et al. Current trends and future scope in 3D printing for surgical management of spine pathologies. Bioprinting 2022;26:e00197.
- Pugliese L, Marconi S, Negrello E, et al. The clinical use of 3D printing in surgery. Updates Surg 2018;70:381-8. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [Crossref] [PubMed]
- Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016;159:1485-500. [Crossref] [PubMed]
- Izatt MT, Thorpe PL, Thompson RG, et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J 2007;16:1507-18. [Crossref] [PubMed]
- Ballard DH, Mills P, Duszak R Jr, et al. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad Radiol 2020;27:1103-13. [Crossref] [PubMed]
- Higgins M, Leung S, Radacsi N. 3D printing surgical phantoms and their role in the visualization of medical procedures. Ann 3D Print Med 2022;6:100057.
- Bagaria V, Chaudhary K. A paradigm shift in surgical planning and simulation using 3Dgraphy: Experience of first 50 surgeries done using 3D-printed biomodels. Injury 2017;48:2501-8. [Crossref] [PubMed]
- Wake N, Rude T, Kang SK, et al. 3D printed renal cancer models derived from MRI data: application in pre-surgical planning. Abdom Radiol (NY) 2017;42:1501-9. [Crossref] [PubMed]
- Yang M, Li C, Li Y, et al. Application of 3D rapid prototyping technology in posterior corrective surgery for Lenke 1 adolescent idiopathic scoliosis patients. Medicine (Baltimore) 2015;94:e582. [Crossref] [PubMed]
- Zhao W, Shen C, Cai R, et al. Minimally invasive surgery for resection of ossification of the ligamentum flavum in the thoracic spine. Wideochir Inne Tech Maloinwazyjne 2017;12:96-105. [Crossref] [PubMed]
- Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016;15:115. [Crossref] [PubMed]
- Leary OP, Crozier J, Liu DD, et al. Three-Dimensional Printed Anatomic Modeling for Surgical Planning and Real-Time Operative Guidance in Complex Primary Spinal Column Tumors: Single-Center Experience and Case Series. World Neurosurg 2021;145:e116-26. [Crossref] [PubMed]
- Li C, Yang M, Xie Y, et al. Application of the polystyrene model made by 3-D printing rapid prototyping technology for operation planning in revision lumbar discectomy. J Orthop Sci 2015;20:475-80. [Crossref] [PubMed]
- Tong Y, Kaplan DJ, Spivak JM, et al. Three-dimensional printing in spine surgery: a review of current applications. Spine J 2020;20:833-46. [Crossref] [PubMed]
- Jitpakdee K, Liu Y, Heo DH, et al. Minimally invasive endoscopy in spine surgery: where are we now? Eur Spine J 2023;32:2755-68. [Crossref] [PubMed]
- Sharif S, Afsar A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg 2018;119:472-8. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. [Crossref] [PubMed]
- Parr WCH, Burnard JL, Wilson PJ, et al. 3D printed anatomical (bio)models in spine surgery: clinical benefits and value to health care providers. J Spine Surg 2019;5:549-60. [Crossref] [PubMed]
- Mao K, Wang Y, Xiao S, et al. Clinical application of computer-designed polystyrene models in complex severe spinal deformities: a pilot study. Eur Spine J 2010;19:797-802. [Crossref] [PubMed]