
3D-printed titanium alloy cage in anterior and lateral lumbar interbody fusion for degenerative lumbar spine disease
Highlight box
Key findings
• 3D-printed porous titanium (3DppTi) alloy cages are associated with a 3.03% subsidence rate in lumbar interbody fusion, which is significantly lower than historically published rates for alternative cage types.
What is known and what is new?
• There is limited clinical data assessing the clinical outcomes of patients undergoing lumbar fusion with 3DppTi alloy cages.
• This is the first study assessing fusion rates from an anterior and lateral approach to lumbar interbody fusion using 3DppTi alloy cages.
What is the implication, and what should change now?
• Further matched cohort, or randomized control trials are required to assess for statistically significant improvement in overall fusion rates comparing cage types.
Introduction
Background
Since 2002 in Australia, the Australian Therapeutic Goods Administration has supported the use of titanium or polyetheretherketone (PEEK) cages for use of interbody fusion in spinal surgery, with registration of these devices on the Australian Register of Therapeutic Goods (1). In the lumbar spine, there is evidence that PEEK cages are associated with up to 10% non-union rate and 15% subsidence rate (2). PEEK does not itself directly fuse to bone, but acts as an interbody spacer, whilst bone formation occurs around it. Titanium alloys, which are also commonly used in bone screws and joint replacements are able to directly stimulate bone integration onto the surface, however, traditional interbody cages made of solid machined titanium have been associated with high subsidence rates. This is probably due to their modulus of elasticity (‘stiffness’) being much greater than that of natural bone (3,4).
Combination titanium/PEEK cages (integrated titanium endplates with a PEEK body) have also been studied in anterior lumbar interbody fusion (ALIF) and demonstrate a 5% non-union and subsidence rate, which is comparable to PEEK cages (5,6).
Rationale and knowledge gap
Recently the use of 3D printing for medical implants has become an alternative manufacturing technique that allows for the production of significantly more versatile implants. This technique can augment of reduce the modulus of elasticity in various parts of the implant, a property that is not possible with traditional manufacturing methods. The theoretical clinical advantages of 3D-printed porous titanium (3DppTi) cages are higher rates of fusion at an earlier timepoint and lower rates of subsidence. Preliminary data, initially in ovine models, showed a higher stability and better bone growth compared to PEEK (7). However, there is limited data on their efficacy and safety in humans.
Objective
We aimed to assess the outcome of 3DppTi cages in ALIF and lateral lumbar interbody fusion (LLIF) in our institutional series. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-120/rc).
Methods
Participants and study site
A retrospective chart review was performed of all patients who underwent a LLIF or ALIF with a 3DppTi cage, at the Melbourne Private Hospital in Melbourne, VIC, Australia. All surgeries between January 2020 and February 2021 were included, and were performed by the two senior authors, both of whom are Neurosurgeons with a subspecialty spinal surgery practice. The first senior author (A.M.) has 13-year experience and the second senior author (M.A.) has 7 years of experience in minimally invasive spinal surgery. The cages used were from two different companies; The 4WEB® cage from LifehealthcareTM (Sydney, NSW, Australia) and The Modulus® cage from NuvasiveTM (San Diego, CA, USA). Patients who had the 4WEB® cage implanted, had Allovance Crunch bone allograft from Australian BiotechnologiesTM (Sydney, NSW, Australia) used within the cage and patients who had the Modulus® cage implanted, had synthetic Attrax® bone graft from NuvasiveTM within the cage. The choice of cage was based on surgeon preference, commercial availability of the cage at the time of surgery and the amount of bone graft used was dependent on the size of the central bone graft window in each cage. Cages were sized intra-operatively with the use of a trial device and fluoroscopy. All cages were between 10° and 15° of lordosis and 8–12 mm of height, with no hyper-lordotic cages used. Demographic, radiological and surgical data was collected from all patients.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Royal Melbourne Hospital Human Research Ethics Committee (No. HREC 2021.242) approval was gained for the study to be performed at Melbourne Private Hospitals, with a waiver for written participant consent, given the retrospective nature of the study. Patients who did not have follow-up imaging with computed tomography at 12 months were excluded from the analysis.
Outcomes
For each case, radiological fusion on follow-up imaging was assessed using the criteria set out by Gruskay et al. [2014] (8): presence of boney trabeculation across the fusion level, lack of boney lucency at the graft/vertebral body junction, device subsidence, cystic changes on the endplates or haloing surrounding instrumentation. Radiological fusion was assessed independently by two authors (C.D. and T.S.), with any discrepancy resolved by review and consensus with the senior authors. Any complications, including subsidence or non-union were recorded and reviewed by the senior author (A.M.). If subsidence was present, it was classified using the Marchi grade (9).
Statistical analysis
Statistical analysis of the data was performed using Prism 9TM (GraphPad Software, Boston, MA, USA). A multiple logistic regression analysis was performed and odds ratios were calculated for pre-specified risk factors to assess for effect on subsidence. A P value of less than 0.05 was considered significant.
Results
Patient demographic data
A total of 57 patients were identified as having undergone lumbar spine fusion via a lateral or anterior approach from January 2020 to February 2021. Seven patients were excluded from the analysis due to no follow-up imaging performed, with 50 patients included in the final data set. Baseline characteristics, operative details, follow-up time frame and risk factors for non-union were collated (Tables 1,2). The average age was 61 years at the time of surgery with 44% of patients being male and 56% female. Thirty-two percent of patients were active smokers or had a body mass index (BMI) defined as obese or greater at the time of surgery. Sixteen percent had a pre-operative diagnosis of osteoporosis confirmed on bone densitometry and were on hormone replacement therapy. Twenty-four percent were being treated for type 2 diabetes mellitus. Indications for surgery included adjacent segment disease from previous decompression and/or fusion, spondylolisthesis or foraminal stenosis from degenerative spine disease.
Table 1
| Operative data | ALIF | LLIF |
|---|---|---|
| Spinal levels | ||
| L1/2 | 0 | 1 |
| L2/3 | 0 | 4 |
| L3/4 | 2 | 12 |
| L4/5 | 6 | 17 |
| L5/S1 | 24 | 0 |
| Total levels | 32 | 34 (66 total levels from both approaches) |
| Supplemental posterior fixation (levels) | 20 | 25 |
| NuvasiveTM Modulus cage + Attrax graft | 0 | 27 |
| Life HealthcareTM 4WEB cage + Crunch bone graft | 32 | 7 |
Values are presented as number. ALIF, anterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion.
Table 2
| Demographic data | Value |
|---|---|
| Age (years) | 61 [17–84] |
| Gender | |
| Male | 22 [44] |
| Female | 28 [56] |
| Non-union risk factors | |
| Smoker | 16 [32] |
| Type 2 diabetes mellitus | 12 [24] |
| Osteoporosis | 8 [16] |
| Obesity | 16 [32] |
| Time to follow-up imaging (months) | 11.3 |
| Number of levels | |
| 1 level | 38 |
| 2 levels | 8 |
| 3 levels | 4 |
Values are presented as mean [range], number [%], mean, or number.
Operative data
Fifty patients had a total of 66 levels operated on. Thirty-two levels were from an anterior approach and 34 from a lateral approach. L5/S1 was the commonly operated level, at 36%, followed by L4/5 at 33%. Supplemental posterior fixation was performed on 20 patients (67%) who underwent surgery via an anterior approach and 25 (74%) who underwent surgery via a lateral approach. The 4WEB® cage was used for all patients who underwent fusion via an anterior approach and seven who underwent fusion via a lateral approach. The Modulus® cage was used for the remaining 27 levels for patients who underwent fusion via a lateral approach. The median follow-up time was 12 months, with a range of 7–24 months.
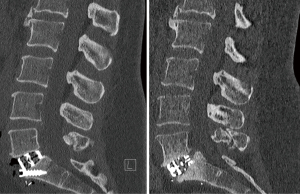
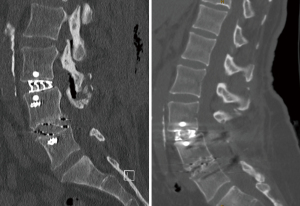
Two patients in the cohort demonstrated subsidence. The first case demonstrated a Marchi grade 0 subsidence at L5/S1, 2 months after an anterior approach with the 4WEB® cage without supplemental posterior fixation, requiring a posterior decompression and fixation due to recurrence of an L5 radiculopathy (Figure 1). This patient remains symptoms free at 2 years follow-up. The second case demonstrated a Marchi grade 1 subsidence from a lateral approach at L3/4 with the Modulus® cage with supplemental posterior fixation, which was asymptomatic and did not require further surgery at 2 years follow-up (Figure 2). An overall subsidence rate of 3.03% was demonstrated. Neither of the patients who demonstrated subsidence did not have any patient risk factors for subsidence.
Statistical analysis
A multiple logistic regression analysis was performed and odds ratios were calculated for the collated risk factors. None of the identified risk factors demonstrated a statistically significant association with graft subsidence (Table 3).
Table 3
| Risk factor | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Smoking | 0.79 | 0.02–28.69 | 0.88 |
| Type 2 diabetes mellitus | 0.63 | 0.01–57.24 | 0.82 |
| Osteoporosis | 0.24 | 0.004–10.02 | 0.43 |
| Obesity | 0.8 | 0.02–57.62 | 0.91 |
Discussion
Key findings
Our results add to the limited literature to date regarding the clinical advantages of 3DppTi cages. We report a 3.03% overall subsidence rate and a 1.5% reoperation rate.
Strengths and limitations
This study is the first to assess fusion and subsidence rates using 3DppTi cages for lumbar fusion from an anterior approach, as well as the first to compare two different approaches in the same cohort.
Limitations of our study are that late subsidence could have been missed due to a relatively short follow-up window, however most subsidence appears to happen within the first few weeks to months post-operatively. Graft material has not been examined directly with 3DppTi cages, which again, is not able to be adequately assessed in our study due to low power. Adl Amini et al. performed a comparative analysis of patients undergoing standalone LLIF with 3DppTi cages compared to PEEK (10). This study did demonstrate a statistically significant improvement in fusion rates in the early, but not late group. However, this study was retrospective and not matched, with different patients in the early and late groups. This may not be applicable to our cohort given 74% of our patients undergoing LLIF had supplemental posterior fixation. Previously the use of recombinant human bone morphogenic protein-2 (rhBMP-2) in grafting has been associated with higher subsidence rates (11), but this was not used throughout our study, as it has not been approved in Australia since 2017. Given the retrospective nature of this study, clinical outcomes were not assessed in a standardised fashion and therefore we were unable to correlate this with our findings of low subsidence and re-operation rate. One study reported no significant difference in Oswestry Disability Index scores at 1 year between the two retrospectively reviewed groups who underwent minimally invasive transforaminal lumbar interbody fusion (TLIF) with PEEK and 3DppTi cages (12). However, this study also found no significant difference in fusion rates between the two cages. Although a randomised control study would be preferable, a larger prospective matched cohort study would be sufficient to elucidate the statistical differences in risk factors for patients undergoing LLIF or ALIF for degenerative lumbar spine disease. The risk factors for subsidence collected have been shown consistently in literature to be associated with subsidence, we did not see this in our data. This may be due to the low overall numbers and therefore underpowered statistical analysis with our series. These patients similarly did not have any surgical risk factors for subsidence such as hyper-lordotic cages.
Comparison with similar researches
Makino et al. assessed the used of 3DppTi cages in posterior lumbar interbody fusion (PLIF) compared to combination (13). This study examined 63 patients who underwent a PLIF at a single institution since 2015. Outcomes of the last 34 patients (39 levels) to undergo PLIF with titanium/PEEK combination cages were compared with the first 29 patients (36 levels) to undergo PLIF with 3DppTi alloy cages. They found that although subsidence rate was significantly less in the 3D-printed cage group at 6 months, there was no difference in subsidence rates at 12 months. There was also no significant difference in the fusion rates at 12 months between the two groups.
A further study assessed the fusion rates of 3DppTi cage implants from a different manufacturer, packed with silicate-substituted calcium phosphate (SiCaP) bone graft, using trans-foraminal and lateral approaches (14). A retrospective chart review of 93 patients (150 levels) of a single surgeon was performed. This study demonstrated a fusion rate of 98.9% and subsidence rate of less than 1%. These figures are better than the reported data on titanium/PEEK cages, however no direct comparison was performed.
A single institution case series study in 2020 was the first to assess subsidence rates in 3DppTi cages in LLIF (15). Twenty-nine patients with 59 levels were included, with an overall subsidence rate of 2/59 cages (3.4%) and 2/29 patients (6.9%), both of whom were asymptomatic and did not require further surgery. A recent study by Alan et al. (16), examining subsidence rates in LLIF in 55 patients using 3DppTi cages, reported a subsidence rate of 8%, with 1.8% of patients requiring re-operation for their subsidence for recurrent symptomatology.
More recently a retrospective series comparing 3DppTi cages compared to PEEK in PLIF surgery found that 3DppTi cages demonstrated a higher fusion rate at 1 and 2 years, however there was no difference in subsidence between the two groups (17). Similarly, a retrospective comparative review of 3DppTi cages compared to solid Titanium cages in TLIF surgery demonstrated a lower rate of subsidence with the 3DppTi cages (18).
Deng et al. has published the only prospective controlled trial comparing 3DppTi cages to PEEK in both cervical and lumbar fusion surgery (19). Their lumbar fusions were performed by a transforaminal approach and assessed 20 patients in each cage. They demonstrated no significant difference fusion and subsidence rates at 3 and 6 months when compared to PEEK cages, however the grade of fusion was significantly better in the 3DppTi cage group.
Many risk factors for subsidence after interbody fusion have been reported in the literature (5,20,21). These include both patient factors; such as osteopenia/osteoporosis, obesity, smoking, age and sex; as well as surgical risk factors, such as supplemental posterior instrumentation, graft/cage size, interbody bone graft materials and end plate preparation/surface area.
The cost of 3DppTi cages used in this study are similar to their PEEK counterparts with both companies for both lateral and anterior cages. This is consistent with the results in a study by Alan et al., which demonstrated an overall superior economic outcome with 3DppTi cages compared to PEEK, when accounting for differences in revision rates (22).
Explanations of findings
Overall, the limited clinical data of 3DppTi cages reports a significant improvement in fusion and subsidence rates when compared to traditional cages.
Implications and actions needed
Although a randomised control study would be preferable, a larger prospective matched cohort study would be sufficient to elucidate the statistical differences in risk factors for patients undergoing LLIF or ALIF for degenerative lumbar spine disease.
Conclusions
This study is the first to report subsidence rates in both LLIF and ALIF surgery using 3DppTi cages. It is also the first to include two cages from separate companies as a comparison. The overall subsidence rate in this study was 3.03% with a reoperation rate of 1.5%. The results of this study are comparable to other publications assessing fusion rates in Lumbar interbody fusion with these cages and adds to the growing data that supports the use of these cages over traditional PEEK and titanium/PEEK cages. There is, however, a need for a larger scale randomised or matched cohort study to further validate these findings.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-120/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-120/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-120/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-120/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Royal Melbourne Hospital Research Ethics Committee (No. HREC 2021.242) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Australia Government, Department of Health. Consultation: Proposed reclassification of spinal medical devices. Version 1.
0. 2019. Available online: https://www.tga.gov.au/sites/default/files/consultation-proposed-reclassification-spinal-implantable-medical-devices.pdf - Behrbalk E, Uri O, Parks RM, et al. Fusion and subsidence rate of stand alone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. Eur Spine J 2013;22:2869-75. [Crossref] [PubMed]
- Seaman S, Kerezoudis P, Bydon M, et al. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J Clin Neurosci 2017;44:23-9. [Crossref] [PubMed]
- Ament JD, Vokshoor A, Yee R, et al. A Systematic Review and Meta-Analysis of Silicon Nitride and Biomaterial Modulus as it Relates to Subsidence Risk in Spinal Fusion Surgery. N Am Spine Soc J 2022;12:100168. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Assem Y, et al. Combination Ti/PEEK ALIF cage for anterior lumbar interbody fusion: Early clinical and radiological results. J Clin Neurosci 2016;34:94-9. [Crossref] [PubMed]
- Assem Y, Mobbs RJ, Pelletier MH, et al. Radiological and clinical outcomes of novel Ti/PEEK combined spinal fusion cages: a systematic review and preclinical evaluation. Eur Spine J 2017;26:593-605. [Crossref] [PubMed]
- McGilvray KC, Easley J, Seim HB, et al. Bony ingrowth potential of 3D-printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J 2018;18:1250-60. [Crossref] [PubMed]
- Gruskay JA, Webb ML, Grauer JN. Methods of evaluating lumbar and cervical fusion. Spine J 2014;14:531-9. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8. [Crossref] [PubMed]
- Adl Amini D, Moser M, Oezel L, et al. Fusion assessment in standalone lateral lumbar interbody fusion: 3D-printed titanium versus polyetheretherketone (PEEK) cages. J Spine Surg 2022;8:323-32. [Crossref] [PubMed]
- Campbell PG, Cavanaugh DA, Nunley P, et al. PEEK versus titanium cages in lateral lumbar interbody fusion: a comparative analysis of subsidence. Neurosurg Focus 2020;49:E10. [Crossref] [PubMed]
- Kim DY, Kwon OH, Park JY. Comparison Between 3-Dimensional-Printed Titanium and Polyetheretherketone Cages: 1-Year Outcome After Minimally Invasive Transforaminal Interbody Fusion. Neurospine 2022;19:524-32. [Crossref] [PubMed]
- Makino T, Takenaka S, Sakai Y, et al. Comparison of Short-Term Radiographical and Clinical Outcomes After Posterior Lumbar Interbody Fusion With a 3D Porous Titanium Alloy Cage and a Titanium-Coated PEEK Cage. Global Spine J 2022;12:931-9. [Crossref] [PubMed]
- Mokawem M, Katzouraki G, Harman CL, et al. Lumbar interbody fusion rates with 3D-printed lamellar titanium cages using a silicate-substituted calcium phosphate bone graft. J Clin Neurosci 2019;68:134-9. [Crossref] [PubMed]
- Krafft PR, Osburn B, Vivas AC, et al. Novel Titanium Cages for Minimally Invasive Lateral Lumbar Interbody Fusion: First Assessment of Subsidence. Spine Surg Relat Res 2020;4:171-7. [Crossref] [PubMed]
- Alan N, Vodovotz L, Muthiah N, et al. Subsidence after lateral lumbar interbody fusion using a 3D-printed porous titanium interbody cage: single-institution case series. J Neurosurg Spine 2022;37:663-9. [Crossref] [PubMed]
- Yang JJ, Kim DM, Park S. Comparison of Fusion, Subsidence, and Clinical Results Between 3D-Printed Porous Titanium Cage and Polyetheretherketone Cage in Posterior Lumbar Interbody Fusion: A Minimum of 2 Years Follow-Up. World Neurosurg 2023; Epub ahead of print. [Crossref]
- Toop N, Dhaliwal J, Grossbach A, et al. Subsidence Rates Associated With Porous 3D-Printed Versus Solid Titanium Cages in Transforaminal Lumbar Interbody Fusion. Global Spine J 2023; Epub ahead of print. [Crossref]
- Deng Z, Zou Q, Wang L, et al. Comparison between Three-Dimensional Printed Titanium and PEEK Cages for Cervical and Lumbar Interbody Fusion: A Prospective Controlled Trial. Orthop Surg 2023;15:2889-900. [Crossref] [PubMed]
- Yao YC, Chou PH, Lin HH, et al. Risk Factors of Cage Subsidence in Patients Received Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine (Phila Pa 1976) 2020;45:E1279-85. [Crossref] [PubMed]
- Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:1268-73. [Crossref] [PubMed]
- Alan N, Deng H, Muthiah N, et al. Graft subsidence and reoperation after lateral lumbar interbody fusion: a propensity score-matched and cost analysis of polyetheretherketone versus 3D-printed porous titanium interbodies. J Neurosurg Spine 2023;39:187-95. [Crossref] [PubMed]


