
Does the use of tranexamic acid intraoperatively reduce postoperative blood loss and complications following biportal endoscopic lumbosacral decompression?
Highlight box
Key findings
• The use of intraoperative tranexamic acid (TXA) upon closure during bi-portal spinal endoscopy for lumbosacral decompression significantly reduces postoperative drainage and blood loss.
• Intraoperative TXA significantly reduced postoperative drainage in single level discectomies while significantly higher drain output was seen in 2 level unilateral laminotomy, bilateral decompression (ULBD) surgeries.
What is known and what is new?
• Postoperative bleeding and epidural hematoma is a known complication after biportal spinal endoscopy for lumbosacral decompression.
• This is the first study investigating the use of intraoperative TXA as an intervention to reduce postoperative drainage and bleeding after biportal spinal endoscopy.
What is the implication, and what should change now?
• The implication of the study is to support the use of intraoperative TXA during biportal spinal endoscopy to reduce the risk of postoperative bleeding
• The use of postoperative drains may not be necessary in certain cases, such as single level discectomies while more indicated for multilevel ULBD cases.
Introduction
Biportal endoscopic spine surgery has recently developed into an effective ultra minimally invasive surgery (MIS) technique for treating common lumbar pathologies such as disc herniations, stenosis, and degenerative disc disease (1-4). Compared to standard MIS techniques, the biportal technique utilizes smaller incisions with less soft tissue dissection, and has been shown to result in improved pain, early mobilization, and shorter length of stay (LOS) (5-8). Multiple clinical studies and meta-analyses have demonstrated excellent clinical results, with low complication rates (9-12). As the biportal technique involves the use of pressurized irrigation fluid and a small working space, intraoperative bleeding is minimized due to the hydrostatic pressure of the constant endoscopic irrigation. Bleeding from the exposed cancellous bone after the laminotomy may be difficult to appreciate until after the irrigation is removed, which may lead to epidural hematoma (13,14). Typically, many surgeons utilize drains post-operatively to reduce this risk of epidural hematoma (15,16). While MIS spine surgery is increasingly utilized in the ambulatory surgery setting, drain usage has been identified as a risk factor for conversion to overnight or inpatient stay (17,18). A strategy to reduce post-operative blood loss could therefore expand the utilization of outpatient biportal spinal endoscopy, an important consideration especially when considering the state of healthcare in the United States.
Tranexamic acid (TXA) is an anti-fibrinolytic agent that has been successfully used in a wide variety of surgical procedures to reduce blood loss and the need for subsequent transfusion with no increase in venous thrombo-embolism or related complications (19-21). TXA has been safely used in spine surgery for decades, with randomized-controlled trials showing similarly reduced rates of calculated blood loss, post-operative drainage and blood transfusion (22-24). Studies have shown the greatest clinical benefit of TXA use in larger, open procedures with or without fusion (25-27). TXA can be administered systematically, i.e., intravenously (IV), or locally via placement in the wound at the beginning or end of a procedure. In multiple studies and meta-analyses, topical TXA has been shown to reduce intra-operative blood loss, post-operative drain output or blood loss, LOS and even the incidence of surgical site hematomas (28-30). Few studies have investigated IV or topical TXA use in minor or MIS procedures. In a randomized controlled study by Elmose et al. of healthy patients undergoing isolated lumbar decompression surgery (of which 50% were MIS), pre-operative IV TXA did not reduce intra-operative blood loss, but did show a reduction in post-operative drain output (31).
No prior studies have investigated the impact of intraoperative TXA use with biportal spinal endoscopy. If systemic intraoperative TXA administration could reduce post-operative drainage and obviate the need for drains following biportal spinal endoscopy, it could enable an increase in ambulatory procedures with significant associated cost-savings for the health system (18). In this present study, we aimed to investigate the impact of IV TXA use on post-operative drainage following biportal spinal endoscopy as an intervention to reduce the need for postoperative drains. We retrospectively analyzed a prospectively collected single institution, single surgeon case series in one of the first studies investigating biportal spinal endoscopy in the United States. We hypothesized that TXA administration will reduce the post-operative drain output and blood loss with no increased risk of post-operative complications or impaired functional outcomes. We present this article in accordance with the TREND reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-129/rc).
Methods
Consecutive patients undergoing biportal spinal endoscopy by a single surgeon at a tertiary care university hospital system in the United States were included in this study. This was a prospectively collected, non-randomized, non-blinded, retrospectively analyzed study and was approved by the University of California Los Angeles Research Administration institutional review board (IRB#22-001674). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants. Inclusion criteria consisted of all primary biportal endoscopic spine surgeries in the lumbar spine with the initiation of the study period in 10/2021 until 6/2023 for the diagnosis of lumbar disc herniation, lumbar stenosis, and lumbar synovial facet cyst causing stenosis requiring surgery for lumbar radiculopathy. Exclusion criteria included any revision surgery and any surgery for the diagnosis of spinal instability, infection, tumor, or trauma. Contraindications for TXA per the manufacturer and the U.S. Food and Drug Administration (FDA) include any patients with subarachnoid hemorrhage, active intravascular clotting, and hypersensitivity to TXA. Patients with any of these contraindications were excluded from this study. Patients were self-selected and scheduled for biportal spinal endoscopy after evaluation by the senior surgeon (D.Y.P.) in the clinic setting using the inclusion and exclusion criteria. All data was collected and obtained in the clinic setting, stored in a secure database and the study was concluded once a minimum follow up of 3 months was obtained. Patients were divided between those receiving a dose of intravenous TXA (1 g) before closure and those that did not receive intravenous TXA. The study was designed with the TXA arm of consecutive cases followed by the non-TXA arm of consecutive cases.
Biportal spinal endoscopy was performed as previously described in prior publications (1,32). Depending on the pathology, lumbar laminotomy with discectomy or unilateral laminotomy, bilateral decompression (ULBD) was performed utilizing the biportal endoscopic technique. Hemostatic agents such as gelatin-thrombin matrix sealants and bone wax were utilized in all cases for control of bony bleeding surfaces during surgery. All patients had a drain placed into the laminotomy site intraoperatively and the postoperative drain output was recorded prospectively. For 2 level surgeries, 2 separate drains were placed, one into each laminotomy site to ensure adequate drainage of each level. Per protocol, patients were either discharged home either on the same day or the next day after surgery after an overnight stay in the hospital. The drains were maintained until their time of discharge. For patients that were discharged home on the same day of surgery, the drains were removed just prior to discharge. For those patients that stayed beyond the same day, the drains were removed prior to discharge. As part of the study protocol, the drains were maintained if the drain output was greater than 50 cc in an 8-hour shift. Patients were not discharged until they met the drain output criteria and clinical criteria for discharge including adequate pain control, baseline ambulation status, and medical stability.
All patients completed previously validated patient-reported outcomes (PROs) of visual analog scale (VAS) for back and leg pain, and the Oswestry disability index (ODI) at the initial preoperative visit and at each subsequent postoperative visit. All patients were also required to report any post-operative complications including neurological changes such as recurrent pain, radicular symptoms, and motor weakness at all points in the follow-up period. The follow-up intervals were 6 weeks, 3 months, 6 months, and 1 year after surgery. Patients were contacted by members of the study team via telehealth visits if there were any missed follow-up intervals for up to 2 years following the index procedure. Patient’s demographic and perioperative data, complications, and PROs were prospectively collected and stored in a secure institutional database. Certain aspects of the demographic and perioperative data were retrospectively collected for the purposes of this study such as estimated blood loss (EBL), body mass index (BMI), American Society of Anesthesiologists (ASA) and Charleston Comorbidity Index (CCI) scores. EBL was subjectively determined by the senior surgeon and recorded by the surgical team in the electronic medical record. The primary outcome was post-operative drain output. Secondary outcomes were post-operative complications and the changes in PROs. Demographic and surgical data were also compared between inpatient and outpatient groups.
Statistical analysis
Analysis was done using a two tailed Student’s t-test after ensuring normal distributions. For skewed, nonparametric distributions, continuous variables are presented as median and interquartile range (IQR), and analyzed using the Wilcoxon rank-sum test, if paired, or Mann-Whitney rank-sum tests if unpaired. Chi-squared tests were used for categorical analysis, with Yates’ continuity correction applied. Visual inspection and the Shapiro-Wilk test was used to assess for normality, with P<0.10 for the latter indicative of non-normally distributed data. If the data were normally distributed, 95% confidence intervals to estimated using standard error. When data were nonparametric, median and 95% confidence intervals were generated using 1,000 bootstrapped samples. EBL and drain output were analyzed using log-normal linear regression after ensuring good fit with a log-normal distribution using a quantile-quantile plot. EBL has been analyzed in other settings using a log-normal distribution (33). Where appropriate, Bonferroni correction was used to adjust for multiple statistical testing. Statistical analyses were performed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Eighty-four patients were identified as having met inclusion criteria. Of them, 45 (54%) patients received TXA and 39 (46%) patients did not receive TXA. Median follow-up was 168 days (IQR, 85–368 days), and follow-up was significantly longer in patients receiving TXA (median 313 days; IQR, 132–406 days) compared to no TXA (median 127 days; IQR, 76–169 days; P=0.00048). There were no differences in patient characteristics between groups: patient age, BMI, ASA score and CCI were all similar (Table 1). Difference in surgical duration was not significant after adjusting for multiple testing. There was no difference in the LOS (P=0.23) or duration a drain was left in place (P=0.62) following surgery.
Table 1
| Variable | No TXA (n=39) | TXA (n=45) | Total (n=84) | P value† |
|---|---|---|---|---|
| Female | 12 (30.8) | 13 (28.9) | 25 (29.8) | >0.99 |
| Age (years) | 62.9±16.2 | 57.5±16.4 | 60.0±16.4 | 0.13 |
| BMI (kg/m2) | 27.3±4.2 | 27.9±4.9 | 27.6±4.6 | 0.49 |
| ASA score >2 | 14 (35.9) | 18 (40.0) | 32 (38.1) | 0.87 |
| Charlson Comorbidity Index | 2.4±2.0 | 2.1±1.8 | 2.2±1.9 | 0.38 |
| Surgery duration (min) | 111±40.3 | 137±48.7 | 125±47 | 0.010 |
| Estimated blood loss (mL) | 1.8±4.4 | 3.5±7.5 | 2.7±6.3 | 0.20 |
| Length of stay (days) | 0.41±0.75 | 0.64±1.00 | 0.53±0.89 | 0.23 |
| Drain duration (days) | 0.48±0.60 | 0.42±0.58 | 0.45±0.59 | 0.62 |
Data are presented as n (%) or mean ± standard deviation. †, adjusted threshold for statistical significance P<0.0063. TXA, tranexamic acid; BMI, body mass index; ASA, American Society of Anesthesiology.
The cases performed included 67 single-level and 17 two-level decompressive procedures spanning from L1 to S1, with no statistically significant differences between the two cohorts in the number of levels (one vs. two) or the distribution of the specific levels addressed (Table 2). The most common level was L4–5 (50%), followed by L5–S1 (21%) and L3–4 (20%). Slightly more than half of patients had a primary diagnosis of stenosis (57%) compared to disc herniation (43%). Consequently, ULBDs were performed more slightly more frequently than discectomies. The primary diagnosis and procedure performed were similar between TXA and no TXA patients (P=0.92 for both).
Table 2
| Variable | No TXA (n=39) | TXA (n=45) | Total (n=84) | P value† |
|---|---|---|---|---|
| Number of levels, n [%] | 0.83 | |||
| 1 level | 32 [82] | 35 [78] | 67 [80] | |
| 2 levels | 7 [18] | 10 [22] | 17 [20] | |
| Levels addressed‡, n [%] | 0.87 | |||
| L1–2 | 1 [2] | 1 [2] | 2 [2] | |
| L2–3 | 5 [11] | 3 [5] | 8 [8] | |
| L3–4 | 9 [20] | 11 [20] | 20 [20] | |
| L4–5 | 21 [46] | 29 [53] | 50 [50] | |
| L5–S1 | 10 [22] | 11 [20] | 21 [21] | |
| Primary diagnosis, n [%] | 0.92 | |||
| Stenosis | 23 [59] | 25 [56] | 48 [57] | |
| Disc herniation | 16 [41] | 20 [44] | 36 [43] | |
| Primary procedure, n [%] | 0.92 | |||
| Discectomy | 16 [41] | 20 [44] | 36 [43] | |
| ULBD | 23 [59] | 25 [56] | 48 [57] |
†, adjusted threshold for statistical significance, P<0.0125; ‡, total number of levels addressed for no TXA is 46, TXA is 55, and 101 for all patients. TXA, tranexamic acid; ULBD, unilateral laminotomy, bilateral decompression.
We found no difference in EBL between TXA and no TXA groups (P=0.20, Table 1). The total drain output was significantly lower in the TXA group compared to the no TXA group (P=0.0028) with an estimated decrease of 31.5 mL in drain output in the TXA group (Table 3). The discectomy group had significantly less drain output as compared to the decompression group and single level biportal surgeries had significantly less drain output as compared to two level surgeries (Table 3). Upon further subanalysis, biportal discectomy and single level surgeries had significantly less drain output with TXA as compared to the no-TXA group (Tables 3,4). ULBD and two level biportal surgeries were associated with significantly higher drain output (Table 3). Although the ULBD subanalysis did not reach statistical significance, the median value of drain output for the TXA group was less than the no-TXA group. In addition, the drain output was significantly lower in single level unilateral biportal surgeries as compared to bilateral (Table 5). No statistical differences were seen between the 2-level unilateral and bilateral surgeries but this is due to the low number of unilateral surgeries and the median drain output for two level bilateral cases were highest of all the groups (Table 5). There were no post-operative blood transfusions in either group.
Table 3
| Comparison | Group | N | Median [IQR] | Difference (95% CI)† | P value‡ |
|---|---|---|---|---|---|
| TXA use | No TXA | 39 | 50 [30–95] | 31.5 (13.4–49.7) | 0.0028 |
| TXA | 45 | 30 [10–60] | |||
| Procedure type | Discectomy | 36 | 30 [13–47.5] | 27.6 (9.2–48.2) | 0.0040 |
| ULBD | 48 | 53 [30–119] | |||
| Number of levels | 1 level | 67 | 30 [15–65] | 68.8 (37.0–117.9) | 0.00019 |
| 2 levels | 17 | 95 [50–155] |
†, estimated difference from log-normal model with bootstrapped CI; ‡, adjusted threshold for statistical significance P<0.0167. IQR, interquartile range; CI, confidence interval; TXA, tranexamic acid; ULBD, unilateral laminotomy, bilateral decompression.
Table 4
| Group | Statistic | TXA | No TXA | Difference (95% CI)† | P value‡ |
|---|---|---|---|---|---|
| Discectomy | n | 10 | 16 | 31.2 (15.7–48.9) | 0.00223 |
| Median [IQR] | 18 [5–33] | 45 [28–80] | |||
| ULBD | n | 25 | 23 | 24.5 (−8.8–57.7) | 0.167 |
| Median [IQR] | 50 [30–80] | 71 [30–124] | |||
| 1 level | n | 35 | 32 | 33.3 (19.0–49.9) | 0.000157 |
| Median [IQR] | 25 [6–38] | 43 [30–89] | |||
| 2 levels | n | 10 | 7 | −9.5 (−100.9–71.9) | 0.815 |
| Median [IQR] | 113 [46–135] | 80 [53–173] |
†, estimated difference from log-normal model with bootstrapped CI; ‡, adjusted threshold for statistical significance P<0.0125. TXA, tranexamic acid; CI, confidence interval; IQR, interquartile range; ULBD, unilateral laminotomy, bilateral decompression.
Table 5
| Levels | Statistic | Unilateral | Bilateral | Difference (95% CI)† | P value‡ |
|---|---|---|---|---|---|
| 1 level | n | 32 | 35 | 17.5 (1.5–35.4) | 0.037 |
| Median [IQR] | 15 [5–29] | 40 [25–80] | |||
| 2 levels | n | 4 | 13 | 46.1 (−6.1–118.0) | 0.26 |
| Median [IQR] | 68 [50–84] | 135 [50–190] |
†, estimated difference from log-normal model with bootstrapped CI; ‡, adjusted threshold for statistical significance P<0.0125. CI, confidence interval; IQR, interquartile range.
No significant differences were detected regarding postoperative complications—including transient postoperative radiculitis, weakness, wound complications, or reherniation—between the two cohorts during the postoperative follow-up period (Table 6). All cases of transient postoperative radiculitis resolved by the 6 week point post-operatively with conservative treatment. There was one case of postoperative weakness with grade 4/5 EHL weakness in each cohort that resolved with physical therapy and rehabilitation. The weakness occurred immediately after surgery for both cases and postoperative MRIs were obtained to evaluate for the cause of the weakness, which demonstrated a small epidural hematoma. The diagnosis of epidural hematoma by MRI was confirmed by a radiologist in both cases. No further surgical intervention was undertaken given the small size of the epidural hematoma and both patients were treated conservatively with improvement.
Table 6
| Complication | No TXA (n=39) | TXA (n=45) | Total (n=84) | P value |
|---|---|---|---|---|
| Postoperative radiculitis, n (%) | 6 (15.4) | 9 (20.0) | 15 (17.9) | 0.79 |
| Postoperative weakness, n (%) | 1 (2.6) | 1 (2.2) | 2 (2.4) | >0.99 |
| Wound drainage, n (%) | 0 (0.0) | 1 (2.2) | 1 (1.2) | >0.99 |
| Reherniation, n (%) | 1 (2.6) | 1 (2.2) | 2 (2.4) | >0.99 |
TXA, tranexamic acid.
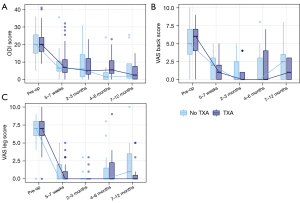
PROs improved significantly from pre-op to most recent follow-up in both groups (Figure 1). In the TXA cohort, median ODI scores improved from 20 to 4 (P<0.0001), median VAS back scores improved from 6 to 0 (P<0.0001), median VAS leg scores improved from 7 to 0 (P<0.0001). In the no TXA cohort, median ODI scores improved from 20 to 2 (P<0.0001), median VAS back scores improved from 5 to 1 (P<0.0001), median VAS leg scores improved from 7 to 0 (P<0.0001). There were no statistically significant differences between cohorts in PROs at any time point post-operatively (Figure 1).
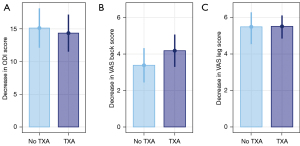
The overall amount of improvement in PROs between inpatient and outpatient cohorts was similar (Figure 2). ODI score decreased by an average of 14.4 points in the TXA group and 15.1 in no TXA group (P=0.71, Figure 2A), while VAS back scores decreased be an average of 4.2 in TXA group and 3.4 in the no TXA group (P=0.22) and VAS leg score decreased by a median of 5.5 in the TXA group and 5.5 in no TXA group (P=0.86, Figure 2B,2C).
Discussion
Biportal endoscopic spine surgery is a novel, ultra-minimally invasive technique that results in improved pain in the immediate post-operative period and shorter hospital stays, but has only recently been adopted in the United States (5-7). In the current, cost-conscious healthcare environment in the U.S., reduction in hospital stays and the increase in ambulatory surgery is increasingly important. The use of IV TXA with this biportal technique may enable further adoption of outpatient biportal surgery by reducing post-operative blood loss and drain use. Here, we conducted one of the first U.S. based studies of biportal endoscopic lumbosacral decompression and found no significant difference in LOS, drain duration, or post-operative complications in cases receiving TXA at closure compared to no TXA. Both groups showed significant, and similar, improvements in PROs.
Notably, TXA use was associated with a significant decrease in post-operative drain output, with an estimated decrease of 31.5 mL compared to no TXA use. In addition, biportal discectomies and single level surgeries had significantly less drain output as compared to ULBD and two-level surgeries. Two level bilateral biportal cases had the highest median drain output, likely due to increased bony cancellous bleeding from each of the two levels. From these results, the use of IV TXA may obviate the need for postoperative drains in single level biportal discectomies, and more necessary in two level bilateral surgeries, a finding that has not yet been reported in the literature. Of course, it is at the surgeon’s discretion to place a drain based on the level of intraoperative bleeding that occurs on a case-by-case basis.
Many studies have highlighted the significant impact of local or systemic TXA use in reducing post-operative drainage and LOS following open spine surgery (26,27,29,34,35). Almost all prior studies evaluating TXA impact have investigated either open decompressions, fusions, or a combination of the two. These studies cannot be directly applied to MIS techniques, either endoscopic and microscopic, as MIS is associated with substantial reductions in post-op drainage compared to open surgery (8,36,37). The only study evaluating TXA use with MIS is a randomized controlled trial (RCT) of 233 patients undergoing MIS lumbar decompression by Elmose et al.; they found a significant reduction in mean post-op drainage of approximately 31 mL with TXA use, similar to that reported here (31). Reductions in post-op drainage may allow patients to return home safely earlier and reduce the risk of neurological deterioration secondary to surgical site hematoma. In study of topical TXA use in 477 patients undergoing open and MIS lumbosacral surgery, McCabe et al. found an absolute reduction in compressive surgical site hematoma necessitating evacuation from 3.3% to 0.38% after initiation of a hospital-wide topical TXA administration protocol (30). Decreases in post-operative drainage and related complications may result in cost savings to the health system, however further study is necessary to evaluate the cost-effectiveness of TXA use in biportal endoscopic surgery (5-7).
Post-operative drain usage is commonly utilized following biportal endoscopic surgery given the relatively high rates of MRI-detected epidural hematoma. Studies performing routine post-operative MRIs have shown epidural hematoma rates of 23.6% to 24.7% following biportal decompression surgery in series of 310 and 158 patients, respectively (13,14). Of note, the overwhelming majority of these epidural hematomas are clinically silent, with only 1.2% to 1.9% of patients undergoing revision surgery for hematoma evacuation in these studies. Compared to endoscopic discectomy, the risk of epidural hematoma is increased in ULBD as a larger laminotomy defect must be created to access contralateral nerve roots, exposing more bleeding cancellous bone. Our results correlate well with this as the drain output was significantly less in biportal discectomies as compared to ULBDs. Interestingly, the use of TXA did not significantly affect the drain output with ULBDs and two-level surgeries, making other methods of hemostasis important intraoperatively, such as gelatin-thrombin matrix sealants (such as Floseal®) and/or bone wax to control bony bleeding, both of which were utilized in all cases in this study. The use of these measures have shown significant reduction in the rate of epidural hematoma following biportal endoscopic decompression by 49% (16). These hemostatic measures were standardized in the procedure to investigate TXA as the study variable. Further study is needed to evaluate if the administration of TXA at various time points in surgery can further reduce the post-operative epidural hematoma risk.
As TXA was given in this study before closure, we did not seek to evaluate the impact of TXA on intra-operative blood loss. In studies involving open spine procedures, TXA administered systemically before incision or locally following incision has been shown to significantly reduce intra-operative blood loss and, in some cases, shorten operative time (23,28,29). This benefit may not translate to MIS procedures. In the previously described RCT, Elmose et al. found no difference in intra-operative blood loss or operative time between those receiving TXA or placebo (31). While we found no difference in EBL in this study, intra-operative blood loss is difficult to estimate in endoscopic spine surgery as bleeding is both limited by the positive pressure of the endoscopic fluid and subsequently quickly diluted (38). Furthermore, pre- and post-operative labs were not routinely drawn for the smaller procedures described here, and we did not calculate blood loss using described standardized methods and formulas (39). Due to the hydrostatic pressure, constant irrigation inflow and outflow within the working space, and meticulous radiofrequency ablation of epidural bleeding, intraoperative bleeding is typically minimal and not an important determinant of postoperative clinical outcomes or significant risk factor for complications. The half-life of intravenous TXA is 2 hours and the rationale behind using TXA near the end of the case was to investigate the effect of TXA after removal of the hydrostatic effect of continuous irrigation. Therefore, the design for this present study at the outset was to investigate IV TXA at closure as an intervention to reduce postoperative bleeding and epidural hematoma risk, which is more clinically relevant and concerning risk factor for potential complications.
Overall, patients in both groups showed significant and similar improvements in ODI, VAS Leg and VAS back scores following biportal decompression. Improvements in PROs in our cohort were similar to those reported in a recent meta-analysis of biportal endoscopic lumbosacral surgery by Park et al., which demonstrated a mean decrease in VAS back scores of 4.1, VAS leg scores of 5.5 and absolute ODI scores of 20 (9). Complication rates were also similar between groups, with low rates of transient post-operative weakness and re-herniation (2.4% each). The most common complication was post-operative radiculitis or dysesthesia (18%), which improved in all patients by six weeks with conservative measures such as NSAIDs and/or oral corticosteroids. Post-operative radiculitis is not a commonly reported outcome and may be associated with inflammation of neural elements. The rate reported here is slightly higher than the 8% rate reported by Alexander & Gardocki in a series of 100 patients undergoing uniportal endoscopic discectomy (40). In this series, biportal endoscopic decompression is a safe and effective procedure and has been shown to result in similar long-term outcomes compared to standard microscopic techniques (5-7).
This study has several limitations. First, this study has a small sample size and therefore we are underpowered to detect differences in rare outcomes or complications. However, our results corroborate well with larger studies in the literature. Similarly, our follow-up duration is relatively short, however most clinical improvement and clinically relevant complications occur within the first 6 months post-operatively. Another limitation is that this is a heterogenous study population of patients who underwent biportal discectomies and decompressions, as well as 1 level and 2 level surgeries, which may influence the results since discectomies and 1 level surgeries have less bleeding risk than decompressions and 2 level surgeries. These were included to analyze the impact of IV TXA within these subpopulations.
Importantly, this is a non-randomized and non-blinded study and TXA was used in roughly the first half of cases chronologically, which resulted in longer post-operative follow-up in the TXA group. As biportal spinal endoscopy is a new technique, TXA was used earlier in the senior author (D.Y.P.) learning curve. As such, TXA case duration tended to be longer on average with greater irrigation volumes, which may contribute to increased postoperative drainage. Despite this, there was still significantly less postoperative drainage with the use of TXA (Table 3). In addition, there were no differences in BMI between the TXA and no-TXA cohorts, making patient habitus less of a contributing factor to the drain output. We would expect the correlation of TXA use with less surgical experience to bias results towards greater drain output, opposite to what is found here. In addition, intra-operative and hidden blood loss was not accurately quantified in this study and immediate pre- and post-operative labs were not collected regularly. This inability to accurately measure EBL by a standardized methodology is a limitation of this study. The main aim of the study was to investigate the use of IV TXA near the completion of the surgery to evaluate the postoperative blood loss and utilize IV TXA as an intervention to reduce the need for drains after biportal surgery. Further investigation is necessary to evaluate the timing of IV TXA throughout the entire perioperative period utilizing standardized methods to measure total blood loss more accurately in biportal endoscopic surgery.
Conclusions
Biportal spinal endoscopy is a promising technique, with particular relevance in the United States with a high cost-conscious healthcare system. In one of the first U.S.-based studies on biportal spinal endoscopy, we found that intraoperative systemic TXA administration was associated with a significant decrease in post-operative drain output, with no impact on the improvement in PROs or rate of post-operative complications. From this, using TXA may obviate the need for postoperative drains in single level biportal discectomies. Larger, well-designed studies are necessary to evaluate the cost-effectiveness of TXA in biportal surgery, especially given the obvious potential for outpatient surgery using the technique. Similarly, the timing of TXA administration, the effect of topical TXA use, and the impact of TXA on total blood loss with biportal spinal endoscopy would all benefit from further investigations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-129/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-129/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-129/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-129/coif). D.Y.P. is a paid consultant for Seaspine, Alphatec, Nuvasive, Amplify Surgical, has royalties for products developed by Seaspine and Alphatec, and owns stock options for Amplify Surgical. D.Y.P. serves on committees for the North American Spine Society and Cervical Spine Research Society. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of California Los Angeles Research Administration institutional review board (IRB#22-001674) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hwa Eum J, Hwa Heo D, Son SK, et al. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine 2016;24:602-7. [Crossref] [PubMed]
- Kim JE, Choi DJ, Park EJ. Clinical and Radiological Outcomes of Foraminal Decompression Using Unilateral Biportal Endoscopic Spine Surgery for Lumbar Foraminal Stenosis. Clin Orthop Surg 2018;10:439-47. [Crossref] [PubMed]
- Liang J, Lian L, Liang S, et al. Efficacy and Complications of Unilateral Biportal Endoscopic Spinal Surgery for Lumbar Spinal Stenosis: A Meta-Analysis and Systematic Review. World Neurosurg 2022;159:e91-e102. [Crossref] [PubMed]
- Heo DH, Lee DC, Kim HS, et al. Clinical Results and Complications of Endoscopic Lumbar Interbody Fusion for Lumbar Degenerative Disease: A Meta-Analysis. World Neurosurg 2021;145:396-404. [Crossref] [PubMed]
- Choi KC, Shim HK, Kim JS, et al. Cost-effectiveness of microdiscectomy versus endoscopic discectomy for lumbar disc herniation. Spine J 2019;19:1162-9. [Crossref] [PubMed]
- Pranata R, Lim MA, Vania R, et al. Biportal Endoscopic Spinal Surgery versus Microscopic Decompression for Lumbar Spinal Stenosis: A Systematic Review and Meta-Analysis. World Neurosurg 2020;138:e450-8. [Crossref] [PubMed]
- Park SM, Lee HJ, Park HJ, et al. Biportal endoscopic versus microscopic discectomy for lumbar herniated disc: a randomized controlled trial. Spine J 2023;23:18-26. [Crossref] [PubMed]
- Park SM, Park J, Jang HS, et al. Biportal endoscopic versus microscopic lumbar decompressive laminectomy in patients with spinal stenosis: a randomized controlled trial. Spine J 2020;20:156-65. [Crossref] [PubMed]
- Park DY, Upfill-Brown A, Nora B, et al. Clinical outcomes and complications after biportal endoscopic spine surgery: a comprehensive systematic review and meta-analysis of 3673 cases. Eur Spine J 2023;32:2637-46. [Crossref] [PubMed]
- Perez-Roman RJ, Gaztanaga W, Lu VM, et al. Endoscopic decompression for the treatment of lumbar spinal stenosis: an updated systematic review and meta-analysis. J Neurosurg Spine 2022;36:549-57. [Crossref] [PubMed]
- Lin GX, Huang P, Kotheeranurak V, et al. A Systematic Review of Unilateral Biportal Endoscopic Spinal Surgery: Preliminary Clinical Results and Complications. World Neurosurg 2019;125:425-32. [Crossref] [PubMed]
- Heo DH, Park DY, Hong HJ, et al. Indications, Contraindications, and Complications of Biportal Endoscopic Decompressive Surgery for the Treatment of Lumbar Stenosis: A Systematic Review. World Neurosurg 2022;168:411-20. [Crossref] [PubMed]
- Kim JE, Choi DJ, Park EJ. Evaluation of Postoperative Spinal Epidural Hematoma After Biportal Endoscopic Spine Surgery for Single-Level Lumbar Spinal Stenosis: Clinical and Magnetic Resonance Imaging Study. World Neurosurg 2019;126:e786-92. [Crossref] [PubMed]
- Kim JE, Choi DJ, Kim MC, et al. Risk Factors of Postoperative Spinal Epidural Hematoma After Biportal Endoscopic Spinal Surgery. World Neurosurg 2019;129:e324-9. [Crossref] [PubMed]
- Wang H, Wang K, Lv B, et al. Analysis of risk factors for perioperative hidden blood loss in unilateral biportal endoscopic spine surgery: a retrospective multicenter study. J Orthop Surg Res 2021;16:559. [Crossref] [PubMed]
- Kim JE, Yoo HS, Choi DJ, et al. Effectiveness of Gelatin-Thrombin Matrix Sealants (Floseal®) on Postoperative Spinal Epidural Hematoma during Single-Level Lumbar Decompression Using Biportal Endoscopic Spine Surgery: Clinical and Magnetic Resonance Image Study. Biomed Res Int 2020;2020:4801641. [Crossref] [PubMed]
- Dodo Y, Okano I, Kelly NA, et al. Risk Factors for Ambulatory Surgery Conversion to Extended Stay Among Patients Undergoing One-level or Two-level Posterior Lumbar Decompression. Spine (Phila Pa 1976) 2023;48:748-57. [Crossref] [PubMed]
- Malik AT, Xie J, Retchin SM, et al. Primary single-level lumbar microdisectomy/decompression at a free-standing ambulatory surgical center vs a hospital-owned outpatient department-an analysis of 90-day outcomes and costs. Spine J 2020;20:882-7. [Crossref] [PubMed]
- Myles PS, Smith JA, Forbes A, et al. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N Engl J Med 2017;376:136-48. [Crossref] [PubMed]
- Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054. [Crossref] [PubMed]
- Duncan CM, Gillette BP, Jacob AK, et al. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty 2015;30:272-6. [Crossref] [PubMed]
- Elwatidy S, Jamjoom Z, Elgamal E, et al. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976) 2008;33:2577-80. [Crossref] [PubMed]
- Raksakietisak M, Sathitkarnmanee B, Srisaen P, et al. Two Doses of Tranexamic Acid Reduce Blood Transfusion in Complex Spine Surgery: A Prospective Randomized Study. Spine (Phila Pa 1976) 2015;40:E1257-63. [Crossref] [PubMed]
- Xie J, Lenke LG, Li T, et al. Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J 2015;15:647-54. [Crossref] [PubMed]
- Choi HY, Hyun SJ, Kim KJ, et al. Effectiveness and Safety of Tranexamic Acid in Spinal Deformity Surgery. J Korean Neurosurg Soc 2017;60:75-81. [Crossref] [PubMed]
- Fatima N, Barra ME, Roberts RJ, et al. Advances in surgical hemostasis: a comprehensive review and meta-analysis on topical tranexamic acid in spinal deformity surgery. Neurosurg Rev 2021;44:163-75. [Crossref] [PubMed]
- Ehresman J, Pennington Z, Schilling A, et al. Cost-benefit analysis of tranexamic acid and blood transfusion in elective lumbar spine surgery for degenerative pathologies. J Neurosurg Spine 2020; Epub ahead of print. [Crossref]
- Shen J, Yang Z, Fu M, et al. The influence of topical use of tranexamic acid in reducing blood loss on early operation for thoracolumbar burst fracture: a randomized double-blinded controlled study. Eur Spine J 2021;30:3074-80.
- Arun-Kumar V, Naresh-Babu J. Is There a Role for Preoperative Local Infiltration of Tranexamic Acid in Elective Spine Surgery? A Prospective Randomized Controlled Trial Analyzing the Efficacy of Intravenous, Local Infiltration, and Topical Administration of Tranexamic Acid. Global Spine J 2021;11:21-7. [Crossref] [PubMed]
- McCabe RW, Tong D, Kelkar P, et al. Preventing Surgical Site Hematoma Using Topical with or Without Intravenous Tranexamic Acid in Lumbosacral Surgery: A Quality Improvement Project. World Neurosurg 2023; Epub ahead of print. [Crossref]
- Elmose S, Andersen MØ, Andresen EB, et al. Double-blind, randomized controlled trial of tranexamic acid in minor lumbar spine surgery: no effect on operative time, intraoperative blood loss, or complications. J Neurosurg Spine 2019; Epub ahead of print. [Crossref]
- Heo DH, Lee N, Park CW, et al. Endoscopic Unilateral Laminotomy with Bilateral Discectomy Using Biportal Endoscopic Approach: Technical Report and Preliminary Clinical Results. World Neurosurg 2020;137:31-7. [Crossref] [PubMed]
- Carvalho JF, Piaggio G, Wojdyla D, et al. Distribution of postpartum blood loss: modeling, estimation and application to clinical trials. Reprod Health 2018;15:199. [Crossref] [PubMed]
- Sun H, Deng L, Deng J, et al. The Efficacy and Safety of Prophylactic Intravenous Tranexamic Acid on Perioperative Blood Loss in Patients Treated with Posterior Lumbar Interbody Fusion. World Neurosurg 2019;125:e198-204. [Crossref] [PubMed]
- Shi P, Wang J, Cai T, et al. Safety and Efficacy of Topical Administration of Tranexamic Acid in High-Risk Patients Undergoing Posterior Lumbar Interbody Fusion Surgery. World Neurosurg 2021;151:e621-9. [Crossref] [PubMed]
- Goldstein CL, Macwan K, Sundararajan K, et al. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine 2016;24:416-27. [Crossref] [PubMed]
- Lee KH, Yue WM, Yeo W, et al. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J 2012;21:2265-70. [Crossref] [PubMed]
- Liu YF, Hong CK, Hsu KL, et al. Intravenous Administration of Tranexamic Acid Significantly Improved Clarity of the Visual Field in Arthroscopic Shoulder Surgery. A Prospective, Double-Blind, and Randomized Controlled Trial. Arthroscopy 2020;36:640-7. [Crossref] [PubMed]
- Smorgick Y, Baker KC, Bachison CC, et al. Hidden blood loss during posterior spine fusion surgery. Spine J 2013;13:877-81. [Crossref] [PubMed]
- Alexander N, Gardocki R. Awake transforaminal endoscopic lumbar discectomy in an ambulatory surgery center: early clinical outcomes and complications of 100 patients. Eur Spine J 2023;32:2910-7. [Crossref] [PubMed]


