
Transforaminal endoscopic thoracic discectomy: surgical technique
Highlight box
Surgical highlights
• Transforaminal endoscopic thoracic discectomy (TETD) is described here as a minimally invasive surgical approach to treating thoracic disc pathology that can be performed in awake patients in an outpatient setting.
What is conventional and what is novel/modified?
• Surgical techniques for treating thoracic disc herniations typically require posterior or lateral transcavitary approaches and often require instrumented fusion.
• TETD is described here as an outpatient, awake, minimally invasive, non-fusion option for treating thoracic disc herniations.
What is the implication, and what should change now?
• The implication of TETD discussed here is that patients can received surgical treatment for thoracic disc herniations with less morbidity and quicker return to life.
Introduction
For surgeons looking to expand their minimally invasive arsenal of techniques, endoscopic spine surgery is particularly attractive because of advantages that include that the procedure can be performed: (I) through a tiny incision (6–8 mm); (II) in awake patients (patient able to converse during procedure); and (III) in an outpatient setting. Many surgeons are attracted to interlaminar endoscopic approaches because they mirror, almost exactly, minimally invasive tubular surgery with the exception that visualization is endoscopic versus microscopic. Transforaminal surgery has a higher barrier to adoption and probably a shallower learning curve (many cases over a long period before mastery is achieved) chiefly due to 2 challenges: (I) needle targeting; and (II) understanding endoscopic visual anatomy (1,2).
Thoracic disc herniations are the result of a weakening of the disc annulus and a bulging or herniation of the nucleus pulposus. Patients can present with a radiculopathy if compression is on the thoracic nerve root or with myelopathy if compression is on the spinal cord itself. Surgical treatment is recommended in cases of radiculopathy refractory to nonoperative treatment and in cases of myelopathy when patients are deemed to be suitable surgical candidates. Thoracic disc herniations represent a surgical challenge because through each possible surgical access corridor exists a significant hazard (3). Thoracic disc pathology is typically ventral to the spinal cord, so posterior approaches are dangerous and ventral and lateral approaches require dealing with pleural contents. Ventral and lateral approaches often also require fusion. Transforaminal endoscopic surgery is performed through such a small tubular retractor (approximately 7 mm) that foraminal and canal pathology can be performed without destabilizing the spine, therefore, avoiding fusion. We present this article in accordance with the SUPER reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-22-109/rc).
Preoperative preparations and requirements
Preoperative preparation and requirements can be divided into 3 parts: (I) patient; (II) surgeon and team; and (III) equipment. First, a suitable patient for transforaminal endoscopic thoracic discectomy (TETD) should be identified. Preoperative discussion should include other options to endoscopic techniques. For procedures performed awake, the patient should be made aware of each of the steps of the procedure to decrease anxiety at the day of surgery. Good patient candidates for the procedure, early on the in the surgeon’s experience, are patients with foraminal disc pathology that is easily approached and removed. Second, the surgeon should have experience with lumbar endoscopic discectomy prior to attempting to treat thoracic pathology. Ideally, a surgeon would review films with another surgeon experienced with TETD prior to surgery. The team will include the surgeon, the anesthesia team member, the surgical technologist, and the circulating nurse. For patients who are undergoing endoscopic spine surgery performed awake with local anesthetic and sedation, the anesthesia team member has a significant role in titrating medication and continuously communicating with the patient so that they are comfortable but not obtunded. The surgical technologist needs to continuously be aware of what is going on at the surgical field and on the video screen to anticipate the equipment needs. The circulating nurse has a large role in maintain the endoscopic tower, fluid, and medication for the procedure. Third, the equipment needed for TETD is that standard for transforaminal lumbar endoscopic spine surgery. An endoscopic drill system should be available to remove the ventral portion of the SAP to allow access to thoracic ventral pathology. A Jamshidi needle can also be helpful for targeting the superior articular process (SAP) for a trans-SAP approach. A radiolucent bed is also required.
Step-by-step description: 2 case examples
Here we present two illustrative cases, one with a surgical video, and a step-by-step surgical technique utilizing a transforaminal endoscopic approach performed through a 6.3 mm working channel endoscope with a 3.8 mm working channel. The surgery demonstrated is performed with the patient awake and in an outpatient setting. The key steps to performing TETD are described.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case example 1
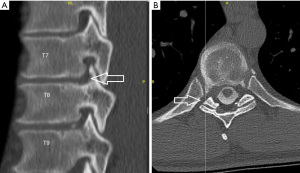
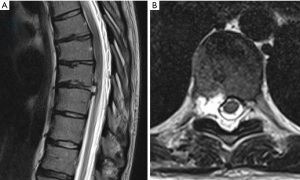
A 32-year-old male presented with a right-sided T7-T8 foraminal disc herniation (Figure 1). He suffered from thoracic back pain and radicular pain despite 1 year of conservative treatment and reported dramatic benefit from a T7–8 selective nerve root block. Pre-operative planning on the T2-weighted axial MR image determined that the optimal approach to access the foramen would require an entry point 7.5 cm off the midline (Figure 1). The patient was taken to the operating room, positioned prone on the Jackson table with hip and chest bolsters. The procedure was performed with intravenous sedation (versed and fentanyl) and local anesthesia (1% lidocaine with epinephrine). An 18-gauge 25 cm spinal needle was used to access the foramen under AP and lateral fluoroscopy targeting the superior corner of the T8 endplate just at the medial border of the T8 pedicle (Figure 1). A 7 mm incision was then made over the needle, a Kirschner wire was placed in the needle, and the needle was removed leaving the Kirschner wire. Sequential tubular dilators were placed over the Kirschner wire and sequential circular reamer drills were placed over the dilators to remove the ventral portion of the T8 superior articulating process. A 7.5 mm outer diameter beveled cannula was then placed in the foramen. The Joimax TESSYS endoscopic system was used for the procedure. Under endoscopic visualization an endoscopic drill was used to drill down the superior articulating process (SAP) and the SAP-pedicle junction to expand the foramen and improve access and visualization of the foraminal and canal anatomy. Figure 1G,1H demonstrate endoscopic camera views of the foramen after drilling and the endoscopic grasper removing the disc herniation. After adequate discectomy and foraminotomy, the patient was asked prior to terminating the procedure the status of his radicular symptoms. The working channel and scope were removed, pressure was held on the incision for 5 minutes, and the wound was closed with a single interrupted suture (surgery time 33 minutes). Immediately after the procedure the patient noted complete resolution of his radicular symptoms. At his 6-month and 1-year post-operative visit he describes his pre-operative back and radicular pain as completely resolved. A postoperative CT was performed which demonstrates the bony removal after reaming and endoscopic drilling (Figure 2).
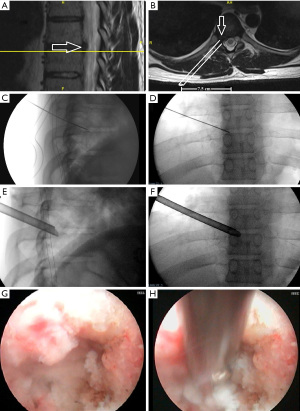
Case example 2
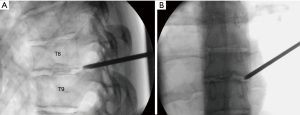
A 31-year-old female presented with a right-sided T8–9 herniated disc (Figure 3) and over 1 year of thoracic back pain and a thoracic radiculopathy referable to the T8–9 dermatomal region on the right despite extensive physical therapy and interventional pain management. Pre-operative planning on the T2-weighted axial MR image determined that the optimal approach to access the foramen would require an entry point 6.0 cm off the midline. The patient was positioned prone on the radiolucent Jackson table with hip and chest bolsters and the procedure was performed under local analgesia and intravenous sedation; the level of anesthetic was titrated, so the patient was able to communicate with the surgeon throughout the procedure. The Joimax TESSYS endoscopic system was used for the procedure. Percutaneous entry was established entering through the skin 6 cm lateral to the midline. Using intermittent fluoroscopic guidance, alternating between lateral and anterior-posterior (AP) view, a 25 cm 18-gauge needle was advanced and placed in the disc space through Kambin’s triangle, between the exiting and traversing nerves. A discogram using omnipaque radio-opaque dye and indigo carmine was performed. Discogram dye is sometimes used to help distinguish disc material from non-disc material in endoscopic spine procedures. A Jamshidi needle was then placed, after the discogram, using the same technique (Figure 4). An AP fluoroscopic view was used to assure that the disc space was entered before the needle was past the medial wall of the pedicle. Sequential reamers were used to enlarge the neural foramen by removing the ventral aspect of the superior facet and 7.5 mm outer diameter beveled cannula was then docked in the foramen. At this point, the endoscope was placed in the cannula, and the remaining portion of the procedure was performed under endoscopic visualization. The key steps are demonstrated in the accompanying video (Video 1) and in Figure 5.
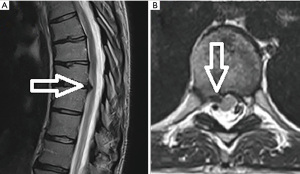
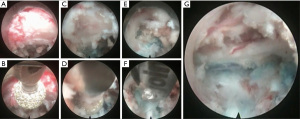
After adequate discectomy and foraminotomy, the patient was asked prior to terminating the procedure the status of her radicular symptoms. The working channel and scope were removed, pressure was held on incision for 5 minutes, and the wound was closed with a single interrupted suture (surgery time 40 minutes). Immediately after the procedure the patient noted complete resolution of her radicular symptoms. At her 6-month and 1-year post-operative visit she describes her pre-operative back and radicular pain as completely resolved. A post-operative magnetic resonance image (MRI) performed at 6 months demonstrated the decompressed foramen and canal (Figure 6).
Postoperative considerations and tasks
Postoperative considerations are reviewed in detail with the patient the day before surgery and given in writing to the patient after surgery. Typically, short course of pain medication and muscle relaxants are given to the patient, but are often not needed. Wound care is minimal because a single dissolvable suture is used to close the wound. The patient may shower on the day of surgery and drive the day after surgery. Often, patients who fly to our centers for surgery, fly home the same day as the surgery. Activity is as tolerated. Patients usually do not follow up in person, but with a phone call. Any concerns about recurrent symptoms are addressed by ordering follow-up imaging. For patients with spinal cord compression, an MRI thoracic is ordered 6 weeks after surgery to assure that the spinal cord remains decompressed.
Tips and pearls
Safety
The authors recommend that surgeons learn and practice in a cadaver laboratory setting prior to attempting TETD. After sufficient practice, a surgeon might consider visiting an experienced endoscopic spine surgeon and observing a TETD. Finally, a surgeon might consider having an experienced surgeon proctor their first TETD. Also, just like with any thoracic spine surgery case, take special precautions to be at the correct operative level.
Planning
We recommend measuring the optimal starting distance from midline using the T2 axial MRI as demonstrated in Figure 1B. This distance should be the maximal distance from the midline to approach the foramen without contacting the ribs or endangering the contents of the thoracic cavity.
Prepare the patient
There are established more invasive approaches to treating thoracic disc herniations (3). The patient should be aware of these prior to considering endoscopic spine surgery. The decision to proceed with TETD should be made with the patient once all other options have been discussed.
Targeting
The surgeon should take sufficient time for accurate and precise needle targeting at the beginning of the procedure. In Case 2 demonstrated here, a Jamshidi needle was used because a spinal needle could not penetrate through bony overgrowth in the foramen. If the needle targeting is not at the medical wall of the pedicle on AP while it is at the superior endplate of the inferior vertebra, the surgeon will not be able to access the canal when the endoscope is brought in. Advanced endoscopic spine surgeon will sometime dock the needle on the SAP, then use an endoscopic drill to enter the foramen. This is the “trans SAP approach” (4).
Foraminoplasty
The thoracic foramen usually needs to be expanded in order to allow entry of the beveled tubular retractor and to allow for adequate visualization of foraminal and canal pathology. The analogy for understanding foraminoplasty is, “opening a window shade to look through a window”. The foraminoplasty is performed in two parts: (I) reaming the SAP; and (II) using the endoscopic drill to drill ventral SAP dorsal to the foramen and to drill the SAP-pedicle junction.
Understanding endoscopic visual anatomy
Once the endoscope is brought into the operative field, the surgeon should take the time to identify key anatomical landmarks before proceeding. The SAP-pedicle junction is one such landmark. A key step should also be to take a ball probe and place it medial to the inferior pedicle and confirm its location on AP fluoroscopy. In Case 2, the surgeon used indigo carmine dye to aid in identifying disc material. The quality of high-definition endoscopes today usually obviates the need to use disc staining dyes to help discern disc material.
Hemostasis
A radiofrequency probe should be available to treat epidural bleeding. Tamponade through the beveled tubular retractor can also be performed as can application of hemostatic agents in the field.
Durotomy
Durotomy can be treated by applying fibrin glue and/or a collagen patch at the end of the case (5).
Relative or absolute contraindications
Foraminal disc herniations are the most easily accessible lesions to treat with TETD. More central disc herniations and large central calcified disc herniations are more challenging to access but are not contraindications for surgeons experienced in endoscopic spine surgery.
Discussion
Other studies have shown that TETD is an effective procedure for treating thoracic disc herniations (6-16), migrated thoracic disc herniations (17), and calcified thoracic disc herniations (17-19). The authors share here 2 case descriptions, an operative video, and a step-by-step technique for performing TETD. The advantages of being able to perform a thoracic discectomy as on outpatient procedure through a tiny incision compared to a thoracotomy are obvious. But there are 2 significant barriers to adopting endoscopic spine surgery: (I) money (i.e., capital equipment purchases that go along with performing endoscopic spine surgery); and (II) developing the technical skills that go along with performing endoscopic spine surgery.
Spine surgery as a service line does not seem to be getting less expensive. Purchases of navigation equipment, robots, and endoscopic towers are expensive capital purchases that offer patients safer, more minimally invasive surgery. The cost of surgery is increasing with these purchases, which can be difficult to justify to hospitals purchasing the equipment when the surgery pays the same. Safer surgery and shorter hospitals stays do make financial sense, and the TETD procedure described here is certainly an example of what could be a surgery with less complications and a shorter hospital stay.
Developing technical skills to perform TETD is a different kind of investment: an investment in surgeon’s time. Most surgeons practicing today have not had the benefit of learning endoscopic spine surgery techniques in residency for in fellowship. Taking time out of practice to attend cadaver laboratories or visit surgeons who are currently performing endoscopic spine surgery is an investment of time. A multicenter randomized controlled trial was published by Gadjradj et al. (20) on the cost-effectiveness of full endoscopic versus open discectomy for sciatica. The results suggest that endoscopic discectomy is more cost-effective from the societal perspective (principally due to expediting getting the patient back to work and back to life).
Conceptually, the authors ask the reader to consider the concept of TETD as natural orifice surgery. The thoracic neural foramen is the natural window to the spinal canal. An endoscope can be placed at an angle between 30 and 45 degrees in foramen, and with a 30-degree oriented cameral, the view is as if one were looking straight into the foramen and directly underneath the ventral epidural space. There are several challenges that go along with, (I) obtaining this idealized view and, (II) performing surgery through this view. We describe here in detail the need for precise targeting and bone removal to establish docking in the foramen. A 30 degree endoscope allows the surgeon to look up into the foramen, but the endoscopic instruments and drill are still straight and do not follow the 30 degree cameral angle. Drill have been developed that articulate to correct for this. Endoscopic graspers are manufactured that go straight down the working channel and then return to their previous up-going or down-going curvature once they are out to the distal end of the working channel. In the video of Case 2, a straight endoscopic drill is used. The surgeon moves the beveled tubular retractor and endoscope out of the foremen, and docks to beveled tubular retractor on the ventral portion of the SAP. The straight drill is then seen removing the ventral portion of the SAP. The surgeon does not move the drill, the surgeon has to move the endoscope and tubular retractor: the drill only goes in and out of the endoscope.
Conclusions
In summary, TETD has it financial and technical challenges. The cases and video presented here demonstrate a step-by-step technique that makes it possible to perform a thoracic discectomy in an awake patient, in an outpatient setting, in a minimally invasive fashion.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-22-109/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-109/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-109/coif). AET serves as an unpaid editorial board member of Journal of Spine Surgery from December 2022 to November 2024. The other author has no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lewandrowski KU, Telfeian AE, Hellinger S, et al. Difficulties, Challenges, and the Learning Curve of Avoiding Complications in Lumbar Endoscopic Spine Surgery. Int J Spine Surg 2021;15:S21-37. [Crossref] [PubMed]
- Wang H, Huang B, Li C, et al. Learning curve for percutaneous endoscopic lumbar discectomy depending on the surgeon's training level of minimally invasive spine surgery. Clin Neurol Neurosurg 2013;115:1987-91. [Crossref] [PubMed]
- Kerezoudis P, Rajjoub KR, Goncalves S, et al. Anterior versus posterior approaches for thoracic disc herniation: Association with postoperative complications. Clin Neurol Neurosurg 2018;167:17-23. [Crossref] [PubMed]
- Hasan S, White-Dzuro B, Barber JK, et al. The Endoscopic Trans-Superior Articular Process Approach: A Novel Minimally Invasive Surgical Corridor to the Lateral Recess. Oper Neurosurg (Hagerstown) 2020;19:E1-E10. [Crossref] [PubMed]
- Telfeian AE, Shen J, Ali R, et al. Incidence and Implications of Incidental Durotomy in Transforaminal Endoscopic Spine Surgery: Case Series. World Neurosurg 2020;134:e951-5. [Crossref] [PubMed]
- Bae J, Kim J, Lee SH, et al. Comparative Analysis of Transforaminal Endoscopic Thoracic Discectomy and Microscopic Discectomy for Symptomatic Thoracic Disc Herniation. Neurospine 2022;19:555-62. [Crossref] [PubMed]
- Bae J, Chachan S, Shin SH, et al. Percutaneous Endoscopic Thoracic Discectomy in the Upper and Midthoracic Spine: A Technical Note. Neurospine 2019;16:148-53. [Crossref] [PubMed]
- Bae J, Chachan S, Shin SH, et al. Transforaminal endoscopic thoracic discectomy with foraminoplasty for the treatment of thoracic disc herniation. J Spine Surg 2020;6:397-404. [Crossref] [PubMed]
- Choi G, Munoz-Suarez D. Transforaminal Endoscopic Thoracic Discectomy: Technical Review to Prevent Complications. Neurospine 2020;17:S58-65. [Crossref] [PubMed]
- Gibson RDS, Wagner R, Gibson JNA. Full endoscopic surgery for thoracic pathology: an assessment of supportive evidence. EFORT Open Rev 2021;6:50-60. [Crossref] [PubMed]
- Guo C, Zhu D, Kong Q, et al. Transforaminal Percutaneous Endoscopic Decompression for Lower Thoracic Spinal Stenosis. World Neurosurg 2019;128:e504-12. [Crossref] [PubMed]
- Moraes Amato MC, Aprile BC, Esteves LA, et al. Full Endoscopic Thoracic Discectomy: Is the Interlaminar Approach an Alternative to the Transforaminal Approach? A Technical Note. Int J Spine Surg 2022;16:309-17. [Crossref] [PubMed]
- Shen J, Shaaya E, Bae J, et al. Endoscopic Spine Surgery of the Cervicothoracic Spine: A Review of Current Applications. Int J Spine Surg 2021;15:S93-S103. [Crossref] [PubMed]
- Telfeian AE, Jasper GP, Oyelese AA, et al. Technical considerations in transforaminal endoscopic spine surgery at the thoracolumbar junction: report of 3 cases. Neurosurg Focus 2016;40:E9. [Crossref] [PubMed]
- Wagner R, Telfeian AE, Iprenburg M, et al. Transforaminal Endoscopic Foraminoplasty and Discectomy for the Treatment of a Thoracic Disc Herniation. World Neurosurg 2016;90:194-8. [Crossref] [PubMed]
- Liu W, Yao L, Li X, et al. Percutaneous endoscopic thoracic discectomy via posterolateral approach: A case report of migrated thoracic disc herniation. Medicine (Baltimore) 2019;98:e17579. [Crossref] [PubMed]
- Gao S, Wei J, Li W, et al. Full-Endoscopic Transforaminal Ventral Decompression for Symptomatic Thoracic Disc Herniation with or without Calcification: Technical Notes and Case Series. Pain Res Manag 2021;2021:6454760. [Crossref] [PubMed]
- Houra K, Saftic R. Transforaminal Endoscopic Discectomy for Large, Two Level Calcified, Thoracic Disc Herniations With 5-Year Follow-up. Neurospine 2020;17:954-9. [Crossref] [PubMed]
- Houra K, Saftic R, Knight M. Five-Year Outcomes After Transforaminal Endoscopic Foraminotomy and Discectomy for Soft and Calcified Thoracic Disc Herniations. Int J Spine Surg 2021;15:494-503. [Crossref] [PubMed]
- Gadjradj PS, Broulikova HM, van Dongen JM, et al. Cost-effectiveness of full endoscopic versus open discectomy for sciatica. Br J Sports Med 2022;56:1018-25. [Crossref] [PubMed]


