
Long-term outcomes following lumbar total disc replacement with M6-L
Introduction
Low back pain is a common cause of morbidity affecting up to 16% of the adults (1). Lumbar interbody fusion and lumbar total disc replacement (LTDR) are effective treatments for degenerative disc disease (DDD) that have failed non-surgical therapies in appropriately selected patients (2). The benefits of motion preserving technologies with LTDR include potentially reducing the degree of adjacent segment disease (ASD) (3,4). LTDRs are indicated to treat DDD at the index surgical level, as well as in the management of ASD when used in conjunction with a fusion, typically above the fusion level as a hybrid procedure (5).
There are over a dozen LTDR implants in use internationally (6). Long-term follow-up is needed for these devices to substantiate their continued use and contribute to the advancement of lumbar arthroplasty technology. The M6-L total disc replacement (TDR) (Orthofix Medical Inc., Lewisville, TX, USA) is a lumbar viscoelastic disc replacement that aims to mimic a physiologic intervertebral disc. M6-L received Conformitè Europëenne (CE) Mark Approval for implantation in the European Union and Australia in 2006.
M6-L allows for six degrees of freedom of movement. Ultra-high molecular weight polyethylene fibers are wound in multiple layers around a polycarbonate urethane (PCU) core bonded to the titanium alloy endplates (7). This is designed to simulate the nucleus pulposus and annulus fibrosis of a normal physiologic disc. A PCU polymer sheath surrounds the core to minimize tissue growth and minimize wear debris. The device is a single unit and wear debris testing has been performed up to 20 million cycles, equivalent to 20 years (7).
Over 18,000 M6-L devices have been implanted worldwide between 2009 and 30 June 2021. There are few long-term follow-up studies on the M6-L device nor data available about the effectiveness of lumbar disc arthroplasty over the longer (5–10 years) term. We note recent findings in cervical disc arthroplasty that suggested the presence of mid-term wear induced osteolysis in patients implanted with the M6-C replacement (8). Therefore, this study additionally investigates whether these findings were also observed with the lumbar M6-L device.
The aim of this study was to evaluate the clinical and radiographic outcomes of a retrospective cohort of patients who underwent LTDR with M6-L as stand-alone TDR procedures, as well as in conjunction with anterior lumbar interbody fusion (ALIF) in hybrid procedures and comment on the effectiveness and durability of the device. We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-22-36/rc).
Methods
All consecutive patients who underwent LTDR with M6-L between January 1, 2011, and January 1, 2021, were included in the study. This cohort of 60 patients represented a single center 10-year experience of two senior spine surgeons with this device.
Inclusion criteria were patients with single, two, or three level painful DDD that had failed non-surgical therapy for at least 6 months. Patients with deformity, instability, moderate to severe facet arthropathy, reduced bone mineral density (BMD) and more than three-level of pathology were excluded from treatment with the M6-L. A decision tree describing surgical intervention for ALIF, TDR or both is presented in Figure 1.

A vascular surgeon performed the anterior retroperitoneal approach in all cases. All patients were implanted with the M6-L device or in combination with a caudal adjacent segment ALIF.
A retrospective chart review recorded preoperative, 6-week and 6-month patient reported outcome measures (PROMs) with final PROMs obtained at most recent follow-up via phone survey or face to face consultation. Back pain visual analog scale (VAS Back) and leg pain visual analog scale (VAS Leg) assessed pain, Oswestry disability index (ODI) assessed disability and Quality of life was assessed via the 12-Item Short Form Survey (SF-12) (7,9). North American Spine Society Patient Satisfaction Index (NASS PSI) evaluated patient satisfaction.
Flexion and extension lumbar radiographs were obtained at most recent follow-up. Device range of motion (ROM) were measured using Inteleviewer Cobb-angle (Intelerad Medical Systems Incorporated) from the superior endplate of the upper vertebra to the inferior endplate of the lower vertebra. The ROM was calculated by subtracting flexion angles from extension angles.
The study reviewed basic patient demographics, clinical adverse events [infections, persisting radicular pain, neurological deficit, deep vein thrombosis (DVT)/pulmonary embolism (PE)], occurrence of reoperations at the index and adjacent levels, and radiographic adverse events [heterotopic ossification (HO) and ASD].
Statistical analysis
Mean, IQR, standard deviation and a 95% confidence interval (CI) were calculated to assess for confounding. Paired two-tailed t-test and Fisher’s exact p-tests were used to assess for clinically significant differences between PROM groups and patients with dynamic radiographs against those without. Statistical significance was assessed at P=0.05. Analyses were performed using the RStudio Service (Version 4.1.2).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients included were provided with a standard privacy disclosure stating that their information will be used for ongoing evaluation of outcomes and that their identity will be protected in any publication arising from this. None of the people included expressed that they did not accept this. Consistent with provisions for low-risk research outlined in the Australian National Health and Medical Research Council’s National Statement on Ethical Conduct in Human Research (2007, updated 2018), this project was reviewed by an independent expert in Human Research Ethics to ensure it met all ethical standards.
Results
Sixty patients underwent LTDR with the M6-L with a mean age of 41 (16–17 years). Most patients (n=42, 70.0%) had a hybrid procedure, 16 (26.7%) underwent standalone LTDR, and 2 (3.3%) a 3-level procedure. Twenty-three (38.3%) patients were lost to follow-up. Thirty-seven (61.7%) were followed for a mean of 4.3 [1–10] years with 36/37 reviewed at a minimum of 2 years and 13/37 followed for over 5 years (Table 1). Thirty (50.0%) patients consented for a final radiographic follow-up (Figure 2).
Table 1
| Variable | 6-month follow-up (n=60) | Final follow-up (n=37) |
|---|---|---|
| Male, n (%) | 38 (63.3) | 22 (59.5) |
| Age, years (mean, median, IQR, SD) | 41, 41, 11.5, 11 | 42, 41, 12, 10 |
| Follow-up time in years, median [IQR] | 4 [4.2] | 4 [2] |
| Hybrid procedures, n (%) | 42 (70.0) | 25 (67.6) |
| Anatomical level, n (%) | ||
| L3/4 | 3 (5.0) | 2 (5.4) |
| L4/5 | 58 (96.7) | 36 (97.3) |
| L5/S1 | 44 (73.3) | 28 (75.7) |
| Operative levels, n (%) | ||
| 1 | 16 (26.7) | 10 (27.0) |
| 2 | 42 (70.0) | 25 (67.6) |
| 3 | 2 (3.3) | 2 (5.4) |
TDR, total disc replacement; ALIF, anterior lumbar interbody fusion; SD, standard deviation; IQR, interquartile range.
Patient reported outcomes
Preoperative mean VAS back, VAS leg, ODI, and SF-12 (physical/mental) showed improvements postoperatively at 6 weeks and again at 6 months postoperative. PROMs showed statistically significant improvements (P<0.05) from baseline to last follow-up (Table 2). There was no difference in age, sex and PROMs between followed-up (n=37) and unfollowed patients.
Table 2
| Variable | Baseline (SD) | 6 weeks (SD) | 6 months (SD) | Final follow-up (SD) | P value (95% CI) |
|---|---|---|---|---|---|
| VAS Back | 8.1 (0.97) | 5.2 (0.97) | 3.0 (1.5) | 1.8 (2.3) | 2.2×10−16 (5.43 to 7.12) |
| VAS Leg | 7.4 (1.9) | 4.4 (2.0) | 2.7 (2.0) | 2.2 (3.2) | 4.9×10−11 (4.11 to 6.43) |
| ODI | 29 (4.5) | 20 (5.0) | 10 (5.0) | 6.7 (10.0) | 3.3×10−14 (18.4 to 25.9) |
| SF-12 Physical | 26 (3.1) | 38 (5.3) | 45 (5.3) | 50 (11.0) | 6.2×10−16 (−27.9 to −20.8) |
| SF-12 Mental | 49 (8.5) | 55 (8.5) | 56 (6.8) | 58 (6.5) | 1.9×10−6 (−12.6 to −5.95) |
VAS, visual analogue scale; ODI, Oswestry disability index; SF-12, 12-point short form survey; CI, confidence interval; SD, standard deviation.
The patients available for additional follow-up were very satisfied with the operation. Using the NASS PSI, 31 patients (83.8%) reported a score of 1 indicating that the procedure met their expectations. Four patients indicated a score of two and two individual patients recorded a score of 3 and 4.
Complications and reoperations
The overall rate of complications was 22% (13/37) in this series. Four superficial and 1 deep infection required antibiotics but not surgery. Acutely (<6 months) 5 patients suffered persisting radicular pain which resolved (4 patients) or required nerve root steroid injections (1 patient). One patient required a spinal cord stimulator for persistent neuropathic leg pain. One patient suffered continuing radicular pain (>6 months) managed by pain specialists. Only 1 patient suffered a DVT.
Only 1 patient required reoperation at the index TDR level: a 60-year-old man with osteopenia (femoral neck T-score of −1.15) underwent an uneventful hybrid procedure with day-2 postoperative CT showing appropriately positioned protheses. Five days postoperatively he suffered severe back pain. CT demonstrated subsidence at both levels of the M6-L and ALIF cage. The patient was instrumented posteriorly from L4 to S2 without removal of the LTDR (Figure 3).
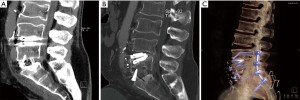
Two patients had pseudoarthrosis requiring posterior fixation at the L5-S1 ALIF level (and did not require revision at L4-5 TDR level).
Radiographic findings
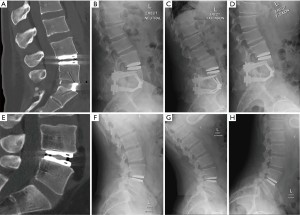
Thirty (50%) patients consented for a final radiographic follow-up. There was no difference in PROMs at 6 weeks and 6 months between patients that obtained final follow-up X-rays and those who did not (all P>0.05). There were no identified device failures or migrations noted on final dynamic radiographs. All 30 patients maintained motion at the index operative level with a mean ROM of 8.6 (0.41 to 17) degrees on flexion and extension radiographs. There was a downtrend in index level ROM over the follow-up time of 1 to 10 years (Figure 4). Day 2 postoperative CT lumbar scans compared to later 10-year follow-up dynamic radiographs for a hybrid patient and single level LTDR patient are shown in Figure 5.
Eight patients demonstrated adjacent segment degeneration, but no patient had symptomatic ASD requiring reoperation. Only two patients demonstrated clinically significant HO (class 3), whilst 8 patients (class 2), 11 patients (class 1), and 9 patients (class 0) (Table 3).
Table 3
| Complications | Number of events |
|---|---|
| Adjacent segment degeneration | 8 |
| Adjacent segment disease | 0 |
| Heterotopic ossification [McAfee classification (0–4)] | |
| 0 | 9 |
| 1 | 11 |
| 2 | 8 |
| 3 | 2 |
| 4 | 1 |
Discussion
First generation LTDR implants were designed based on knee and hip, steel and ball arthroplasty technology (11). However, these modular devices lack the natural compressibility and elasticity of a physiologic intervertebral disc (12). Such devices include the Maverick (Medtronic), Charite (DePuy Spine Inc), Prodisc-L (Centinel Spine) and ActivL (Aesculap) (13).
The second generation TDR implants were designed mimic a physiologic intervertebral disc and are typically monobloc implants (14). These implants include the Freedom lumbar disc (AxioMed), the LP ESP (FH Orthopedics), and the M6-L (Spinal Kinetics). There are only two studies reporting outcomes with the M6-L device. Schätz et al. [2015] described two years follow-up of 83 patients that underwent lumbar arthroplasty with M6-L reporting significant improvement at 24 months postop in ODI and VAS (7). There were no revisions, device removals, or serious adverse device related events. Byvalstev et al. [2021] described a small series of 11 athletes that underwent lumbar arthroplasty with M6-L reporting significant improvement of ODI and SF-12 with mean follow-up of 3.18 years (15).
The final long-term follow-up for Activ-L and Prodisc-L lumbar arthroplasty devices was published in 2021 (16). This represents the two most rigorously studied LTDR devices. The mean preoperative VAS back pain was 7.9 for activL and 7.8 for ProDisc-L which was similar to our mean preoperative VAS back pain of 8.1 with M6-L. At three years follow-up, the mean VAS back had improved to 2.5 for Prodisc and 2.2 for ActivL, similar to our study with mean VAS back improvement to 1.8 at 4.3 years follow-up.
Our study reported statistically significant improvements also in VAS leg, ODI, and SF-12 (physical and mental) postoperatively which were sustained and demonstrated continued improvement to mean follow-up of 4.3 years. The improved PROMs in our study support the findings of previous M6-L reports and those demonstrated in the long-term results of the IDE trial for ActivL and Prodisc-L (7,15).
The M6-L demonstrated long-term effectiveness and durability. We found no device failures and no evidence of the presence of mid-term wear induced osteolysis. There is only one reported case of M6-L device failure in the literature (14). No patient developed ASD that required surgical intervention. Combined analyses of the FDA trial for ActivL and Prodisc-L demonstrated a reoperation rate of 5.0% at 7 years (16). We report an index level reoperation rate of 1.7% with M6-L in a 60-year-old male with osteopenia who suffered L4/5 M6-L delayed subsidence and required posterior instrumentation without removal of the LTDR. This index level reoperation secondary to reduced bone density was early in our operative experience with LTDR a decade ago. Osteopenia (T score <−1.0) and definitely osteoporosis (T score <−2.5) are now well recognized contraindications to LTDR; however, patients over 60 years old may be considered for LTDR if normal BMD and without circumferential spinal stenosis (13).
We found lumbar arthroplasty with M6-L resulted in high patient satisfaction consistent with mid- to long-term results with the Prodisc-L (17). The majority (94.6%) of our patients responded as NASS PSI of 1 or 2, indicating they would undergo the same operation again for the same result. Two patients responded with a higher NASS PSI score which was more attributable to the access procedure. One patient who reported a NASS PSI of 3 suffered a deep wound infection and another patient who reported a score of 4 suffers from leg pain and substance abuse.
Heterotopic ossification remains a long-term concern in spinal arthroplasty due to adverse effects on motion preservation and clinical outcomes (18). In our study only 3 patients demonstrated clinically significant HO comprising McAfee class III (two patients) and class IV (one patient) (6,10).
We had a radiographic loss to follow-up of 50%. Patients declined dynamic radiographs secondary to COVID-19 restrictions, busy lifestyle, and risks of radiation exposure. These patients reported a positive improvement in PROMs with no significant difference observed between patients who obtained radiographs, and those who did not.
Long-term motion of arthroplasty devices raises concern regarding wear debris. No patient had adverse device events directly attributable to wear debris in our study. Arthroplasty devices are rigorously tested in laboratory simulators to determine measurement of wear debris prior to development. There are reported cases of detected wear debris during revision arthroplasty (19-22). Wear debris has been shown to induce inflammatory, vascularization, and innervation growth factors (22).
Of the 30 patients who received long-term radiographic follow-up, all had preserved motion on flexion and extension films. The average ROM was 8.6 degrees. There was a decline in device ROM over time to long-term follow-up. The M6-L showed maintenance in motion up to 10 years.
The M6-L device was a completely assembled one piece device that allowed insertion without requiring separate insertion of a polymer insert. This lack of modularity may lower potential device related failures by reducing the number of articulating surfaces. In our experience, this device provided increased ease of insertion without producing an unintended focal deformity. The 10 degrees lordotic elastic memory of the M6-L maintained a segmental lordotic angle while being semi-constrained on non-physiologic excessive motions in all 6 degrees of freedom. Additionally, the viscoelastic design allowed for additional motion preservation as it relates to shock dampening compressive forces (13). Further prospective studies are needed to determine if this provides additional protection for ASD as compared to non-compressible arthroplasty devices.
Future directions
Whilst we did not directly assess value as one of the outcomes of this project, this is increasingly important in the context of value-based healthcare. Future work will include an economic analysis of the costs involved in LTDR that is linked to the PROMs. There is limited literature about the use of clinically important improvement (CII) measures in spinal surgery, but Power et al. [2021] warned against the use of generic CII across different diagnoses in spine surgery but usefully established a comprehensive set of criteria for future studies. In their study the authors were able to derive cost-effectiveness using the incremental cost-utility ratio and showed that although patients in the surgical group had higher expected costs, they had better expected outcomes and concluded that early surgery was cost-effective when compared with non-surgical care (23). Applying a similar methodology to our series in the future will provide important additional information to support health payer subsidies for the LTDR procedure.
Limitations
This study has several limitations as a mid-sized single institution case series. The study size is limited and is not multi-center. However, this represents two senior surgeons experience both past their learning curve with LTDR. We note the heterogeneity of this study and that inclusion of patients with hybrid constructs may limit the generalizability of our study. However, including these patients is important given the concern for long-term follow-up for device related failures. This series conducted dynamic radiographic analysis at final follow-up, ASD is best assessed with MRI (24). This restricts the applicability of our ASD findings, and we suggest future studies utilize MRI at follow-up.
This study’s extended follow-up period showed a radiographic loss to follow-up of 50% and final PROMs loss to follow-up of 38%. We found no difference in PROMs between patients that consented to final follow-up X-rays and those who declined X-rays. Ideally further studies with a larger sample size should be considered. However, research suggests that a loss to follow-up of up to 60% can be acceptable (25).
Conclusions
Lumbar TDR with M6-L showed clinically and statistically significant improvement in PROMs that were sustained at long-term follow-up. There were no osteolysis induced device related failures. Only one index level reoperation needing supplemental posterior instrumentation for subsidence. The device ROM was maintained and showed a downward trend over the 10-year study follow-up period. The NASS PSI demonstrated a high rate of patient satisfaction. The M6-L device was an effective and durable arthroplasty device in this series. Moreover, further multi-center studies should be considered to assess the long-term efficacy past a follow-up of 10 years.
Acknowledgments
The authors wish to thank Professor Nik Zeps for his advice on the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-22-36/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-36/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-36/coif). GMM has disclosures of Globus Medical (consultancy), Device Technologies (travel), LifeHealthcare (travel), National Surgical (travel), and SeaSpine (travel). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients included were provided with a standard privacy disclosure stating that their information will be used for ongoing evaluation of outcomes and that their identity will be protected in any publication arising from this. None of the people included expressed that they did not accept this. Consistent with provisions for low risk research outlined in the Australian National Health and Medical Research Council’ s National Statement on Ethical Conduct in Human Research (2007, updated 2018), this project was reviewed by an independent expert in Human Research Ethics to ensure it met all ethical standards.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dennis D, Tampin B, Jacques A, et al. The prevalence of back pain in patients in one Australian tertiary hospital population. Musculoskeletal Care 2018;16:112-7. [Crossref] [PubMed]
- Sandhu FA, Dowlati E, Garica R. Lumbar Arthroplasty: Past, Present, and Future. Neurosurgery 2020;86:155-69. [Crossref] [PubMed]
- Formica M, Divano S, Cavagnaro L, et al. Lumbar total disc arthroplasty: outdated surgery or here to stay procedure? A systematic review of current literature. J Orthop Traumatol 2017;18:197-215. [Crossref] [PubMed]
- Jacobs WC, van der Gaag NA, Kruyt MC, et al. Total disc replacement for chronic discogenic low back pain: a Cochrane review. Spine (Phila Pa 1976) 2013;38:24-36. [Crossref] [PubMed]
- Atkins T, Coric D, Yue JJ, et al. Lumbar Disc Arthroplasty. Cham: Springer International Publishing, 2017:357-70.
- Abi-Hanna D, Kerferd J, Phan K, et al. Lumbar Disk Arthroplasty for Degenerative Disk Disease: Literature Review. World Neurosurg 2018;109:188-96. [Crossref] [PubMed]
- Schätz C, Ritter-Lang K, Gössel L, et al. Comparison of Single-Level and Multiple-Level Outcomes of Total Disc Arthroplasty: 24-Month Results. Int J Spine Surg 2015;9:14. [Crossref] [PubMed]
- Scott-Young M, Rathbone E, Grierson L. Midterm osteolysis-induced aseptic failure of the M6-C™ cervical total disc replacement secondary to polyethylene wear debris. Eur Spine J 2022;31:1273-82. [Crossref] [PubMed]
- Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 2008;8:968-74. [Crossref] [PubMed]
- McAfee PC, Cunningham BW, Devine J, et al. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 2003;16:384-9. [Crossref] [PubMed]
- Errico TJ. Lumbar disc arthroplasty. Clin Orthop Relat Res 2005;106-17. [Crossref] [PubMed]
- Frost BA, Camarero-Espinosa S, Foster EJ. Materials for the Spine: Anatomy, Problems, and Solutions. Materials (Basel) 2019;12:253. [Crossref] [PubMed]
- Büttner-Janz K, Guyer RD, Ohnmeiss DD. Indications for lumbar total disc replacement: selecting the right patient with the right indication for the right total disc. Int J Spine Surg 2014; [Crossref] [PubMed]
- Lazennec JY. Lumbar and cervical viscoelastic disc replacement: Concepts and current experience. World J Orthop 2020;11:345-56. [Crossref] [PubMed]
- Byvaltsev VA, Kalinin AA, Aliyev MA, et al. Clinical-Instrumental Results and Analysis of Functional Activity Restoration in Professional Athletes After Lumbar Total Disk Replacement. World Neurosurg 2021;151:e1069-77. [Crossref] [PubMed]
- Radcliff K, Zigler J, Braxton E, et al. Final Long-Term Reporting from a Randomized Controlled IDE Trial for Lumbar Artificial Discs in Single-Level Degenerative Disc Disease: 7-Year Results. Int J Spine Surg 2021;15:612-32. [Crossref] [PubMed]
- Siepe CJ, Heider F, Wiechert K, et al. Mid- to long-term results of total lumbar disc replacement: a prospective analysis with 5- to 10-year follow-up. Spine J 2014;14:1417-31. [Crossref] [PubMed]
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med 2002;43:346-53. [PubMed]
- Baxter RM, Macdonald DW, Kurtz SM, et al. Severe impingement of lumbar disc replacements increases the functional biological activity of polyethylene wear debris. J Bone Joint Surg Am 2013;95:e751-9. [Crossref] [PubMed]
- Gornet MF, Burkus JK, Harper ML, et al. Prospective study on serum metal levels in patients with metal-on-metal lumbar disc arthroplasty. Eur Spine J 2013;22:741-6. [Crossref] [PubMed]
- Punt IM, Austen S, Cleutjens JP, et al. Are periprosthetic tissue reactions observed after revision of total disc replacement comparable to the reactions observed after total hip or knee revision surgery? Spine (Phila Pa 1976) 2012;37:150-9. [Crossref] [PubMed]
- Veruva SY, Lanman TH, Isaza JE, et al. Periprosthetic UHMWPE Wear Debris Induces Inflammation, Vascularization, and Innervation After Total Disc Replacement in the Lumbar Spine. Clin Orthop Relat Res 2017;475:1369-81. [Crossref] [PubMed]
- Power JD, Perruccio AV, Cañizares M, et al. Determining clinically important improvement following surgery for degenerative conditions of the spine analysis of the Canadian Spine Outcomes and Research Network (CSORN) Registry. Can J Surg 2021;64:S1-36. [Crossref]
- Urrutia J, Besa P, Campos M, et al. The Pfirrmann classification of lumbar intervertebral disc degeneration: an independent inter- and intra-observer agreement assessment. Eur Spine J 2016;25:2728-33. [Crossref] [PubMed]
- Kristman V, Manno M, Côté P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol 2004;19:751-60. [Crossref] [PubMed]




