
‘SMART’ implantable devices for spinal implants: a systematic review on current and future trends
Introduction
Intelligent sensor technology is increasingly being integrated into the healthcare system. Innovations in medical sensors represent a paradigm shift towards a more objective, data-driven decision-making process (1-5). This practice is especially important for the care of spinal surgery patients where the efficacy and safety of spinal implants and prostheses pose additional challenges (6). Prior to regulatory approval, spinal constructs are evaluated according to standards relating to their intended use. This may require specifically investigating material-based cytotoxicity, and biocompatibility, undertaking preclinical animal studies as well as static and fatigue testing to assess mechanical performance. While these tests allow an insight into how the implant may perform following implantation, they cannot provide real-time diagnostic and performance data from the final implant while it is implanted in a human (3). This problem is inextricably linked to the design of current spinal implants and so a possible solution lies in radically rethinking the capacity and the potential of these devices: by integrating sensors with spinal implants.
The nomenclature of ‘SMART’ orthopedic devices was coined by Burny who referred to his sensing implants as ‘intelligent’ (7). However, various inventors, engineers, and surgeons explored this idea of combining sensors with surgical implants as early as the 1990s. The preferred term of choice was ‘telemetry’ or ‘telemeterized’ devices which refer to the process of recording and transmitting data, often wirelessly. Whilst this concept has been reviewed in the broader context of orthopedics (7-9), the current review focuses on SMART or telemeterized implants for the spine exclusively. Furthermore, as these sensors have not yet achieved widespread use in clinical practice, the majority of available literature is found within engineering databases and journals. This creates an additional challenge for clinicians to access, interpret and apply these findings to their practice. As such, the purpose of this study is to systematically review the available evidence for the application and potential of SMART spinal implants and present them for use by surgeons and clinicians in the field. We present the following article in accordance with the PRISMA reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-100/rc).
Methods
Search strategy
The following methodology complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist. A search on PubMed, Scopus and Google Scholar was made using the following search terms, “spinal OR spine” and “implants OR prosthesis” and “SMART OR sensor OR accelerometer OR strain-gauge OR telemetry covering all periods of time.
Study selection
Studies were reviewed and included for analysis if they fulfilled the following inclusion criteria: (I) peer-reviewed publication; (II) primary evidence; (III) spinal implants equipped with at least one of the following sensors: strain-gauges, accelerometers, pH probe, thermometer; (IV) implants are designed for in vivo use; (V) implants capable of data logging; (VI) implants have a clear purpose for use in humans.
Studies were removed from analysis if they met the following exclusion criteria: (I) secondary evidence such as systematic reviews or meta-analyses; (II) implants are designed for and used in animals e.g., rodents, baboons, ruminants; (III) implants need to be removed from an in vivo environment for data collection; (IV) implants designed for non-spine applications e.g., knee, hip, spinal cord; (V) sensors or wearables that are not applied to surgical devices e.g. measuring intra-discal pressures.
Data collection
After an initial screening process to discard any duplicate studies, two independent reviewers conducted an eligibility assessment of the studies. The first round of assessments involved a basic review of the abstracts and titles. The second and final round of assessment involved reading through the entire text. Each round was conducted independently by both reviewers. Of the eligible studies, the following data were extracted and tabulated: first authors, publication date, study type, number and type of patients, application/purpose of SMART implant, type of sensor, and type of power supply.
Critical appraisal
Risk of bias assessment was conducted for studies included in our primary analysis using the Office of Health Assessment and Translation (OHAT) risk of bias tool for human and animal studies. Applicable studies were rated on a 4-point scale of definitely-low to definitely-high risk of bias for 11 questions across seven domains: selection, confounding, performance, attrition/exclusion, detection, selective reporting, and other bias.
Results
Included studies
Figure 1 outlines the overall search strategy process and the number of studies included or excluded at each round of screening. A total of 457 articles were found through a preliminary search on PubMed, Scopus, and Google Scholar. After the first round of screening, 55 articles were selected for detailed assessment. After the second round of eligibility assessment, 18 studies were included for a qualitative systematic review (10-27). From this eligibility assessment, 13 studies were excluded because they were non-primary forms of evidence (systematic/literature reviews, meta-analysis), 3 studies were excluded as they involved other forms of measurement such as external wearables and intradiscal pressure sensors, 5 proof-of-concept designs yet to be clinically implemented were also excluded. Sixteen studies were also excluded as they featured topics not pertaining to spine surgery or SMART implants under closer inspection.
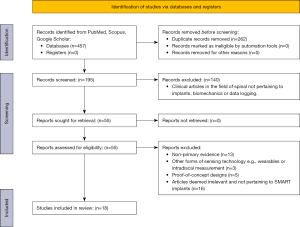
Table 1 shows all relevant outcome measures collected across the 18 included studies including the application and design of the SMART implant. Of the 18 included studies, 8 studies applied sensors on rods or posterior fixators (10-16,27) and 10 studies on vertebral body replacements (VBR) (17-26). Following the temporal course of these studies, SMART rods were investigated and reported on during the 1990s, then, SMART VBRs became widespread in the literature through the late 2000s and early 2010s. All 18 of our included studies utilized strain-gauges as their primary form of sensing technology. There were no reports of accelerometers, thermometers, pH monitors, etc. used clinically and experimentally in human subjects. Pooling across all 18 studies, no more than 20 unique patients were reported to have received a SMART spinal implant.
Table 1
| Study | Study population | Application | Device design | Recorded force on implants |
|---|---|---|---|---|
| Rohlmann et al. (1995) | 1 patient with degenerative instability | Measuring in vivo implant loads after surgery | Dick internal fixators (rods) modified with six strain-gauges, telemetry unit, inductive coil hermetically sealed in a cartridge. | Walking: AF† =−170, BM†=− 2.7 |
| Lifting leg whilst supine: AF =−210, BM =−2.9 | ||||
| Lateral flexion whilst supine: AF =−240, BM =−2.2 | ||||
| Left hand to right knee whilst supine: AF =−125, BM =−1.9 | ||||
| Cycling whilst supine: AF =−245, BM =−3.4 | ||||
| Rohlmann et al. (1997) | 5 lumbar cadaver spines. 3 patients (1 with degenerative instability, 2 with compression fractures) | Comparing in vitro and in vivo implant loads | Modified Dick internal fixators as above | In vitro |
| Standing: AF =−150, BM =−4.5 | ||||
| Flexion: AF =−40, BM =−3.5 | ||||
| Extension: AF =−60, BM =1.5 | ||||
| Patient 1 | ||||
| Standing: AF =−210, BM =−6 | ||||
| Flexion: AF =−220, BM =−6.5 | ||||
| Extension: AF =−270, BM =−6 | ||||
| Patient 2 | ||||
| Standing: AF =−140, BM =−1.5 | ||||
| Flexion: AF =−150, BM =−1.5 | ||||
| Extension: AF =−160, BM =−2 | ||||
| Patient 3 | ||||
| Standing: AF =−130, BM =−6 | ||||
| Flexion: AF =−160, BM =−6.5 | ||||
| Extension: AF =−150, BM =−6.5 | ||||
| Rohlmann et al. (1997) | 1 patient with degenerative instability. 1 patient with a compression fracture | Measuring in vivo loads during walking | Modified Dick internal fixators as above | Relative bending moments |
| From seated position: standing =100%, ventral flexion =105%, extension =107%, lateral bending =108%, axial rotation =108% | ||||
| Whilst standing: sitting down =119%, tip toes =110%, ventral flexion =127%, extension =124%, lateral bending =118%, axial rotation =115%, elevation of extended arm =110%, kneeling on hands and knees =69%, kneeling + flexion =97%, kneeling + extension =80%, kneeling + leg extension =100% | ||||
| Rohlmann et al. (1998) | 2 patients with compression fractures. 1 patient with degenerative instability | Measuring the influence of muscle forces on implant loads | Modified Dick internal fixators as above | Left fixator |
| Relaxed: AF =140, BM =4.2 | ||||
| Abdominal muscle: AF =250, BM =7.2 | ||||
| Back muscle: AF =280, BM =7.2 | ||||
| Pressing: AF =150, BM =5.2 | ||||
| Coughing: AF =145, BM =4.3 | ||||
| Right fixator | ||||
| Relaxed: AF =60, BM =1 | ||||
| Abdominal muscle: AF =55, BM =1.5 | ||||
| Back muscle: AF =190, BM =4.2 | ||||
| Pressing: AF =70, BM =2.4 | ||||
| Coughing: AF =90, BM =2.4 | ||||
| Rohlmann et al. (1999) | 7 patients with compression fractures. 3 patients with degenerative instability | Measuring in vivo loads in different body positions | Modified Dick internal fixators as above | Patient 1‡ |
| Standing: AF (left) =−224, AF (right) =−125, BM (left) =−6.438, BM (right) =−4.143 | ||||
| Sitting: AF (left) =−161, AF (right) =−133, BM (left) =−6.122, BM (right) =−4.239 | ||||
| Supine: AF (left) =−77, AF (right) =−85, BM (left) =−2.464, BM (right) =−0.586 | ||||
| Prone: AF (left) =−102, AF (right) =−89, BM (left) =−3.280, BM (right) =−1.219 | ||||
| Lateral: AF (left) =−72, AF (right) =−95, BM (left) =−3.178, BM (right) =−1.668 | ||||
| Patient 2 | ||||
| Standing: AF (left) =−203, AF (right) =−109, BM (left) =−0.502, BM (right) =−3.769 | ||||
| Sitting: AF (left) =−187, AF (right) =−85, BM (left) =−0.370, BM (right) =-3.271 | ||||
| Supine: AF (left) =−96, AF (right) =−100, BM (left) =0.129, BM (right) =−3.243 | ||||
| Prone: AF (left) =−100, AF (right) =−99, BM (left) =0.257, BM (right) =−3.082 | ||||
| Lateral: AF (left) =−95, AF (right) =−91, BM (left) =0.663, BM (right) =−3.037 | ||||
| Patient 3 | ||||
| Standing: AF (left) =−141, AF (right) =−111, BM (left) =−4.861, BM (right) =−4.610 | ||||
| Sitting: AF (left) =−111, AF (right) =−81, BM (left) =−4.294, BM (right) =−3.927 | ||||
| Supine: AF (left) =−12, AF (right) =−21, BM (left) =−1.239, BM (right) =− 1.345 | ||||
| Prone: AF (left) =−20, AF (right) =−29, BM (left) =−1.538, BM (right) =−1.974 | ||||
| Lateral: AF (left) =−54, AF (right) =−28, BM (left) =−2.222, BM (right) =−1.795 | ||||
| Rohlmann et al. (2000) | 7 patients with compression fractures. 3 patients with degenerative instability | Measuring in vivo loads during sitting, standing, walking, and lying in the first 20 post-operative months | Modified Dick internal fixators as above | Marked interindividual differences in fixator loads |
| Szivek et al. (2005) | 1 patient with spinal deformity | Monitoring fusion progress, measuring strain on spine during cantilever bending | Calcium phosphate ceramic (CPC)-coated strain gauges attached on lamina, uncoated single-element gauge to left rod with a subminiature, remotely powered radio transmitter | Control (non-instrumented) |
| T7 lamina: left =80 μ-strain, right =−180 μ-strain | ||||
| T9 lamina: left =120 μ-strain, right =90 μ-strain | ||||
| T9 vertebral body: left =−405 μ-strain, right =−270 μ-strain | ||||
| T11 vertebral body: left =−320 μ-strain, right =−280 μ-strain | ||||
| Instrumented | ||||
| T7 lamina: left =3 μ-strain, right =−3 μ-strain | ||||
| T9 lamina: left =80 μ-strain, right =2 μ-strain | ||||
| T9 vertebral body: left =−480 μ-strain, right =−190 μ-strain | ||||
| T11 vertebral body: left =−190 μ-strain, right =−280 μ-strain | ||||
| Rod: left =270 μ-strain, right =220 μ-strain | ||||
| PMMA fused | ||||
| T7 lamina: left =−30 μ-strain, right =−390 μ-strain | ||||
| T9 lamina: left =−195 μ-strain, right =−180 μ-strain | ||||
| T9 vertebral body: left =−280 μ-strain, right =−270 μ-strain | ||||
| T11 vertebral body: left =−190 μ-strain, right =−120 μ-strain | ||||
| Rod: left =190 μ-strain, right =230 μ-strain | ||||
| Rohlmann et al. (2008) | 2 patients with compression fractures of L1 | Measuring in vivo loads during sitting, standing, walking, and lying in the first 6 post-operative months | Modified VBR SynexTM, a VBR with a hollow cylinder housing sensor, inductive power coil and telemetry unit | AF for different positions: Lying ≤100, Standing/sitting =150–450, Flexion ≥420, Elevation of arms to 90° with weight ≥700 |
| BM for most exercises ≤2 | ||||
| Rohlmann et al. (2008) | 3 patients with compression fractures of L1 | Measuring in vivo loads during sitting, standing, walking, and lying in the first post-operative month | Modified VBR SynexTM | Relative AF to standing§: standing =100%, flexion =242%, extension =34%, lateral bending =127%, axial rotation =104%, elevation of both arms to 90° =194%, abduction of both arms 90° =108%, walking upstairs =217%, walking downstairs =169%, sitting =99%, sitting + flexion =229%, lying supine =14%, lying prone =22%, lying lateral =26% |
| Relative BM to standing: standing =100%, flexion =272%, extension =37%, lateral bending =246%, axial rotation =133%, elevation of both arms to 90° =203%, abduction of both arms 90° =100%, walking upstairs =219%, walking downstairs =160%, sitting =122%, sitting + flexion =256%, lying supine =45%, lying prone =47%, lying lateral =51% | ||||
| Rohlmann et al. (2010) | 4 patients with A3 type compression fractures | Measuring in vivo loads on spine during whole-body vibration | Modified VBR SynexTM | Maximum force increased with increasing intensity of vibration |
| At maximum intensity vibration (three-axes), the average increase in force on VBR ranges from 123–189% | ||||
| Leaning backwards decreased implant loads to approximately 50% of the force in normal sitting | ||||
| Rohlmann et al. (2011) | 5 patients with A3 type compression fractures | Measuring in vivo loads on spine during sitting | Modified VBR SynexTM | Average change in force whilst sitting: 15° flexion =48% increase, 10° extension =19% decrease |
| Relative reduction in force compared to sitting on stool: bench =7%, stool with padded wedge =9%, knee stool =19%, chair =35%, office chair =41% | ||||
| Rohlmann et al. (2012) | 5 patients with A3 type compression fractures | Measuring in vivo loads on spine during position changes | Modified VBR SynexTM | Maximum force on VBR relative to standing (according to recommendation): lateral to supine to lateral =110%, lateral to prone to lateral =130%, lateral lying to sitting =390%, sitting to lateral lying =395% |
| Maximum force on VBR relative to standing (not according to recommendation): lateral to supine to lateral =425%, lateral to prone to lateral =155%, lateral lying to sitting =405%, sitting to lateral lying =625% | ||||
| Rohlmann et al. (2013) | 5 patients with A3 type compression fractures | Measuring the effect of orthosis on VBR load | Modified VBR SynexTM | Average decrease in resultant force on VBR, lumbo tristep brace =9%, hyperextension orthosis =19% |
| Rohlmann et al. (2013) | 5 patients with A3 type compression fractures | Long-term monitoring up to 65 months | Modified VBR SynexTM | Significant inter-patient variation |
| Force for walking was higher than standing by an average of 100N or 71% | ||||
| Rohlmann et al. (2014) | 5 patients with A3 type compression fractures | Measuring in vivo loads on spine during activities of daily living | Modified VBR SynexTM | Ten activities with the highest resultant force¶: lifting weight from ground =545–1,229 N, forwards arm elevation with weight =611–972 N, moving weight in front of body=758–1,126 N, standing up/sitting down =206–681 N, staircase walking =305–726 N, tying shoes =585–926 N, upper body flexion =341–844 N, lifting a carried weight =261–690 N, washing face =712–831 N, moving from lying to sitting =170–858 N, walking =129–498 N |
| Dreischarf et al. (2015) | 5 patients with A3 type compression fractures | Measuring in vivo loads on spine during forward bending | Modified VBR SynexTM | Maximal force of 450 N measured whilst returning to initial standing position from maximal inclination angle of 53° |
| Flexion during standing (330 N) > flexion during sitting (200 N) | ||||
| Damm et al. (2017) | 5 patients with A3 compression fractures | Measuring in vivo loads on spine and hip during forward bending | Modified VBR SynexTM | Average peak force in VBR during walking =39% of bodyweight |
| Force on implants during walking: hip, knee > spine | ||||
| Barri et al. (2021) | Polymer testing blocks | Monitoring fusion progress | Fowler-Nordheim (FN) sensor data-logger on rods | Decrease in measured voltage corresponds with increasing elastic modulus (modelling fusion) |
†, all AF values are represented in N and BM values are represented in Nm unless otherwise stated; ‡, results from 3 out of 10 patients are reported. Each patient pertains to one of three surgical indications for Dick internal fixators: ‘degenerative instability’, ‘old vertebral fracture’, ‘fresh vertebral fracture’; §, results for 1 patient out of 2 reported; ¶, results for 1 patient out of 5 reported. AF, axial force; BM, bending moment; VBR, vertebral body replacement.
Critical appraisal
The risk of bias for the 18 studies was graded according to the OHAT assessment tool. See Table S1 for the scoring of each study across seven bias domains. The average total score was 30 out of 44. The majority of studies scored relatively low in the selection, confounding, and performance bias domains indicating a high risk of bias in these areas. The majority of studies scored highly in other domains of attrition/exclusion and selective reporting bias indicating a low risk of bias in these areas.
Review of currently published SMART implants
SMART spinal sensors found through our analysis are summarised in Table 2. Pooled across all sources, there are 7 unique designs for spinal implants. To summarise, 3 designs applied sensors on rods (16,27,28) and the remaining 4 designs featured sensors implicated for VBRs (29), pedicle screws (30), interbody cages (31), and orthopedic nails (32) respectively. Five out of 7 designs featured strain-gauges (27-30,33), 1 design used accelerometers (32) and 1 design used both (31).
Table 2
| Source | Design | Power system | Sensor | Intended location |
|---|---|---|---|---|
| Rohlmann et al. | Modified Dick internal fixator | Wireless inductive coupling (passive) | Strain-gauge | Rods/posterior fixators |
| Contains six load sensors, eight-channel telemetry unit, inductive power coil and an antenna | ||||
| Rohlmann et al. | Modified VBR | Wireless inductive coupling (passive) | Strain-gauge | VBR |
| Contains six load sensors, nine-channel telemetry unit, inductive power coil and an antenna | ||||
| Smith et al. | Modified cannulated pedicle screws | Wired | Strain-gauge | Pedicle screws |
| Contains strain gauges and wires within the neck and proximal end of the screw | ||||
| Janna et al. | Cylindrical accelerometer design able to inserted into narrow nails and screws | Undefined | Accelerometer | Orthopaedic nails and screws |
| Contains at least one sensor and microelectronic components including memory device, power supply and communications components | ||||
| Intellirod SpineTM | Titanium/zirconia ceramic, single use strain sensor with radio-frequency transmission | Wireless inductive coupling (passive) | Strain-gauge | Rods |
| Clamps onto rods during kyphotic correction | ||||
| Anderson et al. | Modified tire pressure sensor | Wireless inductive coupling (passive) | Pressure-sensor | Interbody cage |
| MEMS-based technology | Accelerometer | |||
| Compactly contains a load sensor, a two-axis accelerometer, a thermometer and a radio-frequency transmitter | Longevity of 1 year in standby mode | Thermometer | ||
| Barri et al. | Fowler-Nordheim sensor-data-logger attached to polymer model of the spine | Self-powered energy harvesting system based on Fowler-Nordheim quantum tunnelling | Strain-gauge | Rods |
VBR, vertebral body replacement.
Of the 7 designs, 3 are published in the academic literature as primary forms of evidence. In our excluded studies, there was one study reporting on pedicle screws instrumented with strain gauges but yet to be tested on patients during the year 1996 (30). More recently, one study in 2020 published a design of a micro-electromechanical-system-based sensor embedded in an interbody cage (31) which was the first spinal implant design using accelerometers in the last 30 years.
An informal search of sensor technology also revealed unique iterations of SMART spinal sensors which are not found in the initial search of scholarly published literature. These are included in Table 2. In one published patent, an accelerometer sensing package is designed for attachment to an orthopedic implant (32). One permutation of the invention includes an accelerometer, processors, antenna, and a power supply, allowing for the measurement of a physiological acceleration parameter. The intended use of this device is to monitor in vivo bone healing using the acceleration parameter. The sensing package is described to be applied in a variety of ways, located within a medical implant in one embodiment, attached to a wearable device in another, and utilized in a computer-assisted surgery system in yet another embodiment.
From our informal search of commercial websites, we found the LOADPRO Spine Sensing System produced by Intellirod SpineTM that features a unique sensor that clamps onto 5.5 mm rods, allowing surgeons to monitor rod strains during kyphotic correction surgery (33).
Discussion
Systematic review
Objective data is a critical facet of the surgical decision-making process. In the current paradigm of continuous technological advancement, SMART and intelligent devices are becoming more robust and versatile in their ability to play a role in patient monitoring and care. More specifically, improvements to sensor-based technology potentiate a medical care system that prioritizes prevention, early detection, and minimally invasive management of diseases via real-time biofeedback from implantables (34). Furthermore, intelligent sensors that can integrate its collected data with other information such as family history, genomics, and connectomics would create a more personalized and individual-focused healthcare system. Despite these implications for its future use and potential, there is a scarcity of SMART implants designed and trialled for spinal surgery patients. Additionally, to the best of our knowledge, there are no systematic reviews or meta-analyses to date that provide a comprehensive overview of the current state of SMART spinal implants. Furthermore, the available reviews on SMART implants, in general, focus heavily on the engineering and technical aspects of their design and conceptions, with little regard for clinical interpretability and relevance. As such, this systematic review qualitatively addresses the available literature on SMART spinal implants to present clinically relevant and easily understood insights regarding their potential uses and efficacy in both present-day and future spine surgery.
Historical overview of the evolution of SMART implants.
The use of strain gauges to measure the load on a spinal model or cadaveric specimen has historically been a robust and valid technique for in vitro mechanical tests (16,35,36). However, these testing environments are never an exact representation of the in vivo biological environment that spinal prosthetics are placed into. One of the early applications of SMART implants for the spine was the translation of strain-gauges onto spinal rods for in vivo use. This was seen in Waugh’s modification of Harrington rods during spinal deformity correction, where strain-gauges with wires were used to measure the axial load on the implant post-operatively (37). However, this design was problematic due to the infection risks posed by the percutaneous wires (9).
Over the last two decades, the development of wireless, battery-powered systems represent a significant advancement to sensor and telemetry technology (9,38). This improvement would allow the data-logging of prosthetic function over a more dynamic range of everyday patient activities. However, from our systematic review, none of the published incidences of SMART implants featured a battery as such. We posit that this may be due to the following disadvantages of such wireless design: (I) spinal implants such as cages and interbody spacers are too small to be able to house a large, bulky battery; (II) the longevity of most spinal implants would far outlive the short and finite life of the battery; (III) risk of failure and (IV) the hazardous potential of oxidative chemicals.
The majority of SMART spinal implants found for our systematic review feature a passive form of power supply that requires no batteries. This technology is known as inductive coupling, where the SMART implant would be powered by an external ‘coil’ that is attached or worn on the outside (39,40). This external electrical coil is ‘coupled’ or related to an internally placed coil so that the power running through the external coil will transfer to the internal coil and ultimately to the sensor (40). One potential shortcoming of this technology is that the patient needs to wear an external coil to power the sensor and allow data transfer. And whilst these models have been successfully integrated for live biofeedback in cochlear implants and pacemakers, the need to carry or wear external electronics may pose a minor inconvenience for day-to-day use, outside the laboratory setting.
Instrumented Dick internal fixators and VBRs
Fifteen of our included papers involved the seminal work by Rohlmann et al. that designed and tested telemeterized spinal fixation devices, ranging from instrumented Dick internal fixators (Figure 2) and VBRs (Figure 3). An early technical note by Rohlmann et al. in the 1990s lays down some of the founding principles for SMART spinal implants (28). The authors designed a spinal fixation device that housed a special cartridge containing six strain gauges (six channels), capable of measuring the forces and moments acting on the implant in vivo. There are some key characteristics of these implants worth noting: Firstly, the cartridge utilized an ‘inductive power coil’. Secondly, the strain gauges and associated devices such as the power coil and the antennae were hermetically sealed, protecting them from the surrounding biological fluids and tissue.
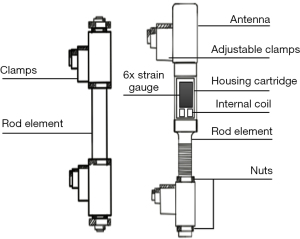
These novel devices were surgically implanted and evaluated in patients as early as 1997. Indeed, Rohlmann et al. compared loads of their six-channel telemeterized implant during in vitro and in vivo axial compression, flexion, extension, and lateral bending (11). Although this initial study utilized a very small sample size (2 cadaveric spine for in vitro and 3 patients for in vivo testing) thus barring any statistical inference, the authors reported an interesting finding that the in vitro and in vivo loads on the implant were vastly different, perhaps due to the lack of muscle forces, abdominal pressures and soft tissue support in cadaveric spines. Ultimately, this study introduced one of the key applications for SMART implants: to collect biomechanical data regarding the efficacy, performance, and safety of spinal constructs in a physiological environment.
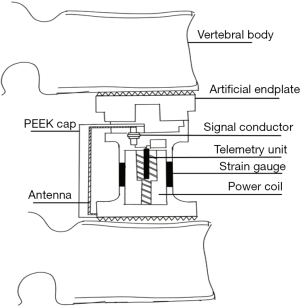
Throughout 1995–2015, the authors conduct several related studies measuring in vivo loads in a variety of different patient contexts such as during walking (12), in different body positions (14), during sitting (20), whole-body vibrations (19), and in general activities of daily living (24) have been published. These recent studies utilized a new design, whereby a VBR is modified to house six load sensors (strain gauges), an inductive power supply, and an antenna for data transmission (29). Furthermore, these studies were clinically relevant as high impact activities that may lead to implant subsidence, pedicle screw loosening or implant failure could be identified. Overall, Rohlmann et al. found that lifting an object from the ground led to the greatest resultant force of 1,650 N on the VBR followed by walking upstairs, upper body flexion, tying shoes, etc. (24). Furthermore, a long-term follow-up of 5 patients revealed that post-operative forces on the ‘SMART’ VBRs varied considerably between patients, suggesting its importance in personalized and nuanced post-operative monitoring and care (23). Additionally, the authors found that monitoring the direct in vivo loads of these VBRs could detect clinically relevant scenarios such as pedicle screw loosening and implant subsidence that were not able to be detected radiographically (23).
These instrumented VBRs were used by other authors. For example, Dreischarf et al. found that forward bending increased the forces on the VBRs of 5 patients to a maximum load of 565 N (25). A study by Damm et al. using the same VBR design and similar patient demographics controlled for the patient’s body weight, reporting that the maximum force in the instrumented VBR was 175% of the patient’s body weight when bending forwards with a 10 kg weight in hand (26). To summarise, the general purpose of the SMART implant designed by Rohlmann et al. and used by others such as Dreischarf et al. and Damm et al. were to collect data necessary to refine implant designs and identify ergonomic activities which pose a high risk of reducing implant stability.
Monitoring fusion progress
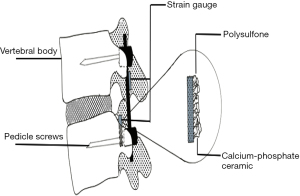
Two of our included studies designed their SMART spinal implant for the specific purpose of monitoring fusion progress (16,27). The design utilized by Szivek et al. was unique, bonding strain-gauges directly to the vertebral components (lamina of T9,10,11) and hardware (left rod) rather than hermetically sealing it within a capsule. Instead, to protect the electrical components of the strain-gauge from the surrounding biological fluids and tissue, the authors coated the lamina-attached strain-gauge with calcium phosphate ceramic (CPC) and allowed it to communicate with the external environment using a remotely powered radio transmitter (Figure 4). Measurements collected for 7 months found that strain on the vertebrae decreased continuously over time, possibly indicating the progression of fusion as the load can be transferred away from the posterior fixation device to the maturing fusion mass (16). Whilst this finding may have been confounded by the gradual detachment of strain-gauge from the lamina over time, it shows a promising application of SMART spinal implants to detect miniature fusion progress that is not able to be detected by current techniques of X-ray, CT, and MRI. Indeed, studies have shown that surgeons cannot confidently assess fusion using these current techniques (41-43). As such, real-time tracking of spinal fusion using SMART spinal devices could be a potential future alternative.
Conversely, several reviews have suggested that SMART spinal devices could be used to detect pseudoarthrosis or other similar post-operative complications such as pedicle-screw loosening, cage subsidence, and general device failure (9,39). Indeed telemeterised VBRs found that the forces on the implant may increase if pedicle screws become loosened or decrease if there is VBR subsidence (22). As such, SMART spinal devices could operate as an early detection and screening tool, allowing surgeons to promptly address these serious post-operative complications before further injury is caused to the patient. An important future step will be correlating the telemeterised information with these clinical findings.
Future directions
As part of our informal analysis of the literature, one study by Anderson et al. presented a novel SMART implant using micro-electromechanical system (MEMS) technology (31). This tiny device was equipped with a pressure sensor, dual-axis accelerometer, and a thermometer and was small enough to be attached to an interbody cage (31). Furthermore, it boasted a very low power consumption relative to other devices in the literature, lasting 36 hours in high-intensity mode and 1 year in low-intensity mode (31). MEMS is a relatively recent technology that amalgamates various mechanical elements, sensors, and electronics on a microscopic semiconductor chip (44,45). The work done by Anderson et al. represents a potential future direction for SMART spinal implants, where MEMS technology would allow engineers to overcome the hurdle of designing devices small enough for a spinal prosthetic. If such designs were to be achieved, SMART implants could be implemented into the cervical spine, which anatomically smaller than the lumbar spine. This would be of great clinical relevance considering the frequency of cervical arthroplasty and anterior cervical discectomy and fusion that is performed each year (46).
Whilst MEMS offers a viable solution to the sizing problem, there is yet a need to address the wireless and battery-free capacity of future SMART spinal devices. Whilst passively-powered inductive coupling seen in our included studies represents an option, it is nonetheless cumbersome and suitable more for laboratory and in-clinic monitoring. There is an abundance of literature that alludes to the potential of energy harvesting as a future option (27,31,39,47,48). Simply put, energy harvesting involves converting the kinetic energy of gross movements such as walking to electrical energy, effectively creating a self-powered system much like a self-winding watch (49).
Our systematic analysis shows an abundance of strain-gauges as the sensing modality of choice. A potential direction for future SMART spinal implants would be the use of accelerometers. Strain-gauges are often disadvantaged by their low accuracy due to confounding environmental factors such as temperature and overuse (50). Furthermore, the calibration process for strain-gauges is often extensive. This puts into question the accuracy of its measurements in a constantly evolving biological environment such as in the spine. We believe that accelerometers might offer a more energy efficient and pragmatic alternative to SMART spinal implants, which have historically been used to measure small and large degrees of movement in the orthopedic context (51).
Finally, this review feature sensors that have been conceptualised and designed for implants in spinal surgery. In the broadest definition, sensors are devices that detects phenomena or changes in the surrounding environment. However, many day-to-day applications of engineering involve an interactive and dynamic system between a sensor and an actuator, a device that can create movement or change in its surroundings. We envision that a truly intelligent SMART spinal implant would not only be able to sense the physical changes in in vivo load sharing but also react to these scenarios and the information that is offered by the sensors. Whilst it is unclear as to what form these actuators may take, it is interesting to imagine that future spinal implants may consist of an adaptive and fluid endoskeleton that may provide additional support or stability when it detects posture or activities that pose a high risk for the spine.
Challenges for SMART spinal implants
Several challenges are facing the widespread integration of SMART spinal devices into the current healthcare system. As discussed previously, a more elegant design of sensors that can function effectively despite being limited by a need for replaceable batteries or external power coil is necessary to allow real-time biofeedback for patients. Furthermore, electronic components should be small enough to be used in conjunction with the relatively small dimensions of spinal devices such as interbody cages, pedicle screws, and VBRs. Lastly, these electronics require a hermetically tight seal to reduce the risk of pyrogenic, cytotoxic and sensitivity reactions to the host whilst being functional.
Secondly, there are obstacles related to their capacity to directly measure the biological load sharing of spinal segments. All designs covered in this review except one discussed by Szivek et al. measure loads exerted on the implant as it performs in vivo. Whilst it may be able to infer the loads exerted on the vertebral body and the posterior elements, these inferences may not correspond to exact forces and moments acting on the in vivo biological spine. As such, future models may explore an alternative design that may be able to obtain quantitative biomechanical evidence from both the implants and the surrounding biological environment.
Thirdly, SMART spinal implants face an economic barrier. Whilst the benefits of early prevention and the associated reduction of costs associated with post-operative complications would provide cost savings for the healthcare system in the long run, current practices of designing and trialling these technologically advanced devices may be too high. Additionally, whilst the FDA is supportive of SMART implant initiatives that aim for live, objective monitoring of patients, the amount of required preclinical evidence and a low benefit-cost ratio for medical device manufacturers can act as barriers in the regulatory pathway (52).
Finally, there are ethical and cybersecurity concerns related to the implantation of SMART, data-logging devices into patients. Indeed, the risk of patient’s privacy being reduced as surgeons gain access to information regarding their activities and other aspects of their biological environment is an impediment to the widespread use of SMART spinal implants. As also discussed by the FDA workshop (52), cyber-attacks and exploits unbeknownst to doctors and the health-care system may also put patients with pacemakers, infusion devices and SMART orthopaedics devices at risk of delayed treatment and diagnosis (52). Furthermore, difficult questions including access to this information, how long it is kept, and the extent to which the patient is informed regarding the implant status must be answered.
Limitations of this study
There are several limitations of this study to consider. Firstly, any systematic or meta-analysis are always limited by the quality of the included studies. Our risk of bias assessment suggests that there are only a few included studies with confidently low risk of bias. This is due to the nature of the studies used to test and validate SMART spinal implants i.e., there is a lack of blinding, no comparison groups, etc. As SMART implants become more widespread and robust, we imagine the scope and validity of clinical trials to improve. The search terms chosen predetermined and somewhat limited the findings as specific sensor types were included. These search terms were chosen based on familiarity with the topic area and to assure that devices that were not specifically called out as SMART, were included in the findings.
Another limitation of this study is the small number of available studies in the literature. Whilst 18 studies were included in the total, we believe that this represents no more than 20 patients who have received SMART spinal implants. The majority of the studies involve the same authors performing a different version of tests on the same group of patients. As such, any pooled findings that are found from our systematic review cannot easily be generalized to the population.
Conclusions
There are a variety of uses for SMART spinal implants. From our systematic review, these intelligent implants have been suggested for use for understanding common activities that may risk damage to implants, monitor the progression of fusion, and early detection for potential complications such as pedicle screw loosening and interbody cage subsidence. Overall, our review found no more than 20 unique patients reported to have received such implants. Nonetheless, several unique designs currently exist in the literature with authors modifying rods, pedicle screws, VBRs, and cages to fit a sensor and its associated electronics. Potential developments in the future lie in the development of miniaturized sensor electronics in the form of MEMS and the widespread use of accelerometers.
Acknowledgments
This work was supported by the Surgical and Orthopaedics Research Lab.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by Guest Editors (Ralph J. Mobbs, Pragadesh Natarajan and R. Dineth Fonseka) for the series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” published in Journal of Spine Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-100/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-100/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-100/coif). The series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” was commissioned by the editorial office without any funding or sponsorship. MHP serves as an unpaid editorial board member of Journal of Spine Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nemčeková K, Labuda J. Advanced materials-integrated electrochemical sensors as promising medical diagnostics tools: A review. Mater Sci Eng C Mater Biol Appl 2021;120:111751. [Crossref] [PubMed]
- Simpson L, Maharaj MM, Mobbs RJ. The role of wearables in spinal posture analysis: a systematic review. BMC Musculoskelet Disord 2019;20:55. [Crossref] [PubMed]
- Chakravorty A, Mobbs RJ, Anderson DB, et al. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: systematic review. BMC Musculoskelet Disord 2019;20:288. [Crossref] [PubMed]
- Stavropoulos TG, Papastergiou A, Mpaltadoros L, et al. IoT Wearable Sensors and Devices in Elderly Care: A Literature Review. Sensors (Basel) 2020;20:2826. [Crossref] [PubMed]
- Witte AK, Zarnekow R. editors. Transforming personal healthcare through technology-a systematic literature review of wearable sensors for medical application. Proceedings of the 52nd Hawaii International Conference on System Sciences; 2019.
- Goel VK, Panjabi MM, Patwardhan AG, et al. Test protocols for evaluation of spinal implants. J Bone Joint Surg Am 2006;88:103-9. [PubMed]
- Burny F, Donkerwolcke M. Smart orthopedic implants. Orthopedics 2005;28:1401-2. [Crossref] [PubMed]
- O’Connor C, Kiourti A. Wireless Sensors for Smart Orthopedic Implants. Journal of Bio- and Tribo-Corrosion 2017;3:1-8.
- Ledet EH, Liddle B, Kradinova K, et al. Smart implants in orthopedic surgery, improving patient outcomes: a review. Innov Entrep Health 2018;5:41-51. [Crossref] [PubMed]
- Rohlmann A, Bergmann G, Graichen F, et al. Telemeterized load measurement using instrumented spinal internal fixators in a patient with degenerative instability. Spine (Phila Pa 1976) 1995;20:2683-9. [Crossref] [PubMed]
- Rohlmann A, Bergmann G, Graichen F, et al. Comparison of loads on internal spinal fixation devices measured in vitro and in vivo. Med Eng Phys 1997;19:539-46. [Crossref] [PubMed]
- Rohlmann A, Bergmann G, Graichen F. Loads on an internal spinal fixation device during walking. J Biomech 1997;30:41-7. [Crossref] [PubMed]
- Rohlmann A, Bergmann G, Graichen F, et al. Influence of muscle forces on loads in internal spinal fixation devices. Spine (Phila Pa 1976) 1998;23:537-42. [Crossref] [PubMed]
- Rohlmann A, Bergmann G, Graichen F. Loads on internal spinal fixators measured in different body positions. Eur Spine J 1999;8:354-9. [Crossref] [PubMed]
- Rohlmann A, Graichen F, Weber U, et al. 2000 Volvo Award winner in biomechanical studies: Monitoring in vivo implant loads with a telemeterized internal spinal fixation device. Spine (Phila Pa 1976) 2000;25:2981-6. [Crossref] [PubMed]
- Szivek JA, Roberto RF, Margolis DS. In vivo strain measurements from hardware and lamina during spine fusion. J Biomed Mater Res B Appl Biomater 2005;75:243-50. [Crossref] [PubMed]
- Rohlmann A, Graichen F, Bender A, et al. Loads on a telemeterized vertebral body replacement measured in three patients within the first postoperative month. Clin Biomech (Bristol, Avon) 2008;23:147-58. [Crossref] [PubMed]
- Rohlmann A, Graichen F, Kayser R, et al. Loads on a telemeterized vertebral body replacement measured in two patients. Spine (Phila Pa 1976) 2008;33:1170-9. [Crossref] [PubMed]
- Rohlmann A, Hinz B, Blüthner R, et al. Loads on a spinal implant measured in vivo during whole-body vibration. Eur Spine J 2010;19:1129-35. [Crossref] [PubMed]
- Rohlmann A, Zander T, Graichen F, et al. Measured loads on a vertebral body replacement during sitting. Spine J 2011;11:870-5. [Crossref] [PubMed]
- Rohlmann A, Petersen R, Schwachmeyer V, et al. Spinal loads during position changes. Clin Biomech (Bristol, Avon) 2012;27:754-8. [Crossref] [PubMed]
- Rohlmann A, Zander T, Graichen F, et al. Effect of an orthosis on the loads acting on a vertebral body replacement. Clin Biomech (Bristol, Avon) 2013;28:490-4. [Crossref] [PubMed]
- Rohlmann A, Dreischarf M, Zander T, et al. Monitoring the load on a telemeterised vertebral body replacement for a period of up to 65 months. Eur Spine J 2013;22:2575-81. [Crossref] [PubMed]
- Rohlmann A, Pohl D, Bender A, et al. Activities of everyday life with high spinal loads. PLoS One 2014;9:e98510. [Crossref] [PubMed]
- Dreischarf M, Albiol L, Zander T, et al. In vivo implant forces acting on a vertebral body replacement during upper body flexion. J Biomech 2015;48:560-5. [Crossref] [PubMed]
- Damm P, Kutzner I, Bergmann G, et al. Comparison of in vivo measured loads in knee, hip and spinal implants during level walking. J Biomech 2017;51:128-32. [Crossref] [PubMed]
- Barri K, Zhang Q, Mehta D, et al. An implantable, battery-free sensing system for monitoring of spinal fusion 2021. 31 p.
- Rohlmann A, Bergmann G, Graichen F. A spinal fixation device for in vivo load measurement. J Biomech 1994;27:961-7. [Crossref] [PubMed]
- Rohlmann A, Gabel U, Graichen F, et al. An instrumented implant for vertebral body replacement that measures loads in the anterior spinal column. Med Eng Phys 2007;29:580-5. [Crossref] [PubMed]
- Smith TS, Yerby SA, McLain RF, et al. A device for the measurement of pedicle screw moments by means of internal strain gauges. J Biomech Eng 1996;118:423-5. [Crossref] [PubMed]
- Anderson WD, Wilson SLM, Holdsworth DW. Development of a Wireless Telemetry Sensor Device to Measure Load and Deformation in Orthopaedic Applications. Sensors (Basel) 2020;20:6772. [Crossref] [PubMed]
- Janna S, Wilson D, Brady P, inventors; Smith & Nephew, Inc., Memphis, TN (US), assignee. Processing sensed accelerometer data for determination of bone healing patent US9445720B2. 2007.
- IntellirodSpine. De Novo Classification Request for LOADPRO Intraoperative Rod Strain Sensors. In: FDA, editor. 2018.
- Andreu-Perez J, Leff DR, Ip HM, et al. From Wearable Sensors to Smart Implants--Toward Pervasive and Personalized Healthcare. IEEE Trans Biomed Eng 2015;62:2750-62. [Crossref] [PubMed]
- Szivek JA, Roberto RF, Slack JM, et al. An implantable strain measurement system designed to detect spine fusion: preliminary results from a biomechanical in vivo study. Spine (Phila Pa 1976) 2002;27:487-97. [Crossref] [PubMed]
- Yu Y, Zhu R, Zeng ZL, et al. The strain at bone-implant interface determines the effect of spinopelvic reconstruction following total sacrectomy: a strain gauge analysis in various spinopelvic constructs. PLoS One 2014;9:e85298. [Crossref] [PubMed]
- Waugh TR. Intravital measurements during instrumental correction of idiopathic scoliosis. Acta Orthop Scand 1966;1-87. [Crossref] [PubMed]
- Ledet EH, D'Lima D, Westerhoff P, et al. Implantable sensor technology: from research to clinical practice. J Am Acad Orthop Surg 2012;20:383-92. [Crossref] [PubMed]
- Karipott SS, Nelson BD, Guldberg RE, et al. Clinical potential of implantable wireless sensors for orthopedic treatments. Expert Rev Med Devices 2018;15:255-64. [Crossref] [PubMed]
- Covic GA, Boys JT. Inductive Power Transfer. Proceedings of the IEEE 2013;101:1276-89. [Crossref]
- Brodsky AE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine (Phila Pa 1976) 1991;16:S261-5. [Crossref] [PubMed]
- Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976) 1993;18:1186-9. [Crossref] [PubMed]
- Kanayama M, Cunningham BW, Weis JC, et al. Maturation of the posterolateral spinal fusion and its effect on load-sharing of spinal instrumentation. An in vivo sheep model. J Bone Joint Surg Am 1997;79:1710-20. [Crossref] [PubMed]
- Terry SC, Angell JB, Barth PW. Silicon Micromechanical Devices. Scientific American 1983;248:44-55. [Crossref]
- Rai-Choudhury P. MEMS and MOEMS Technology and Applications. Bellingham: Bellingham: Society of Photo-Optical Instrumentation Engineers (SPIE); 2000.
- Al Eissa S, Konbaz F, Aldeghaither S, et al. Anterior Cervical Discectomy and Fusion Complications and Thirty-Day Mortality and Morbidity. Cureus 2020;12:e7643. [Crossref] [PubMed]
- Ramakrishna VAS, Chamoli U, Rajan G, et al. Smart orthopaedic implants: A targeted approach for continuous postoperative evaluation in the spine. J Biomech 2020;104:109690. [Crossref] [PubMed]
- D'Lima DD, Fregly BJ, Colwell CW Jr. Implantable sensor technology: measuring bone and joint biomechanics of daily life in vivo. Arthritis Res Ther 2013;15:203. [Crossref] [PubMed]
- Munir B, Dyo V. On the Impact of Mobility on Battery-Less RF Energy Harvesting System Performance. Sensors (Basel) 2018;18:3597. [Crossref] [PubMed]
- Tokyo-Measuring-Instruments-Laboratory. What is strain 2021. Available online: https://tml.jp/e/knowledge/strain_gauge/about.html#:~:text=Strain%20gauges%20are%20provided%20with,a%20strain%20gauge%20is%20used
- Sliepen M, Lipperts M, Tjur M, et al. Use of accelerometer-based activity monitoring in orthopaedics: benefits, impact and practical considerations. EFORT Open Rev 2019;4:678-85. [Crossref] [PubMed]
- Baumann AP, O'Neill C, Owens MC, et al. FDA public workshop: Orthopaedic sensing, measuring, and advanced reporting technology (SMART) devices. J Orthop Res 2021;39:22-9. [Crossref] [PubMed]


