A multicenter study of the 5-year trends in robot-assisted spine surgery outcomes and complications
Introduction
Since their first emergence in the 1990’s, robot-assisted surgical systems have greatly improved and become a means of optimizing the modern operating room (1). Robots have increasingly become incorporated into many surgical fields including gynecology, urology, cardiothoracic surgery, vascular surgery, and general surgery (2-5). The first robot-assisted system for spine surgery, Mazor: SpineAssist®, (Mazor Robotics Ltd., Casesarea, Israel), received FDA clearance in 2004 (6-8). Since then, several others have been approved including Mazor: Renaissance® in 2011; Mazor: X® in 2016; ROSA® Spine (Zimmer Biomet Robotics, Montpellier, France) in 2016, and the Excelsius GPS® (Globus Medical, Inc., Audubon, Pennsylvania) in 2017 (7). Since its inception, robots have been used in spine surgery primarily for placement of thoracic and lumbar pedicle screws.
Since the introduction of robot-assisted spine surgery nearly twenty years ago, a gamut of literature suggests that robot-assisted spine surgeries are safe and can achieve comparable outcomes to conventional techniques (7,9-12). Several studies have shown that robot-assisted surgeries can achieve excellent screw accuracy placement, low intraoperative complication rates, and reduced radiation exposure (10,11,13-20). While these results are encouraging, limitations in supportive studies are hindered by small patient sizes and single-surgeon or single-center series.
This would be the first and largest multi-center study to analyze the trends in outcomes and complications after robot-assisted spine surgery over a five-year period. We hypothesize that the improvements in robot-assisted technology will contribute to considerable improvements in the surgeon’s ability to accurately place screws with robotic-assistance, reliability to obtain registration and robot usage, operative efficiency, and radiation exposure over time. We present the following article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-102/rc).
Methods
Patient selection
Adult (≥18 years old) patients who had undergone robot-assisted spine surgery between 2015 and 2019 at four geographically diverse institutions (Columbia University, Virginia Spine Institute, University at Buffalo Neurosurgery, and University of Virginia Health System) were included. The robotic systems used consisted of the Mazor Renaissance, Mazor X, and Mazor Stealth Edition. A minimum of 25 robot cases were performed per surgeon at each institution. Two institutions (of the four) included all three generation robots (Renaissance, Mazor X, Mazor X Stealth) during the study’s period. Two institutions did not include the Renaissance system. Patients with incomplete data were excluded from this study. All patients included in this study had a minimum follow-up period of at least 90 days after the index surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study, AAAT1470, was approved by the Institutional Review Board of Columbia University Medical Center (No. FWA# 00002636). Written informed consent was exempted because this was a retrospective analysis of de-identified from a multicenter cohort and obtaining informed consent is not required for this type of analysis.
Data collection
Several perioperative factors were compared across the years of surgery, including: patient demographics, comorbidities [e.g., Charlson comorbidity index (CCI), smoking status, obesity], and preoperative diagnosis. Operative factors included open vs. percutaneous surgery, prior spine surgery, total number of instrumented levels, pelvic fixation, interbody fusion, planned vs. executed robot screws, and robot system.
Outcomes of interest included operative efficiency (robot time per screw), radiation exposure (fluoroscopy time per screw), robot complications (e.g., screw exchange, robot abandonment), clinical outcomes [e.g., length of stay (LOS = days between date of discharge and date of admission), 90-day reoperations], and other surgical complications (e.g., dural tear, loss of motor/sensory function, blood transfusion). Robot abandonment was operationally defined as not using the robot to place instrumentation, and relying on fluoroscopy, navigation, or free-hand technique. Total robot time was obtained by when the robot was first brought into the operating field and stopped when the robot was moved out. Similarly, fluoroscopy time was calculated as the amount of time taken to obtain X-rays during screw placement. In all centers, a dedicated research coordinator calculated screw time during OR cases.
Robotic systems
The Renaissance is a 2nd generation robotic system manufactured by Mazor and replaced the SpineAssist in 2011. In comparison to the SpineAssist, the Renaissance had considerable advancements in software and hardware technology. The key elements of the Renaissance system included the proprietary software based on CT images which enabled surgeons to preoperatively plan implant size and trajectories more accurately, several mounting platforms to serve as an interface between the patient and the robotic system, and the renaissance robotic device (RBT), which was a portable computer-controlled platform that spatially positioned and oriented surgical tools in accordance to the preoperative plan.
In 2016, the Mazor X system was introduced and significantly expanded upon the core technology of Renaissance. In comparison to the Renaissance, the Mazor X platform includes a more sophisticated three-dimensional analytical software to improve preoperative planning as well as allow for intraoperative planning (“scan & plan” method) if a preoperative CT scan is not available. The Mazor X planning software allows for better preoperative identification of unique dysplastic features using three-dimensional identification of the patient’s anatomy. In addition, surgeons are able to inspect implant size and trajectory in all three planes simultaneously.
Furthermore, the Mazor X Align application allows surgeons to better plan for spinal deformity cases. This application integrates standing spine X-rays with CT-scan to provide simulations of the impact of corrective changes, such as osteotomies, on spinal alignment in sagittal and coronal planes. The intraoperative guidance system uses an integrated three-dimensional camera with spatial tracking, a surgeon control panel in the sterile area, and a robotic arm which is designed to be serial, rather than parallel, in order to allow for a larger unrestricted range of motion and reduce the need for additional surgical tools. The three-dimensional camera maps the patient and the surrounding environment to produce a three-dimensional image of the operative field. This allows the robot to better self-detect its location and potentially reduce collision with the patient or other elements in the surgical field. Finally, the Mazor system further enhances precision and accuracy through the use of the Mazor X Eye camera to intraoperatively verify the appropriate surgical arm position and trajectory prior to instrumentation at each level.
More recently, the Mazor X Stealth Edition was introduced in 2018. This robotic system integrates Medtronic’s Stealth navigation technology into the Mazor X platform. Using Stealth navigation technology, surgeons have a real-time 3-D visualization as they drill and/or place their implants down the preoperatively planned trajectories. This live visual feedback was designed to provide further predictability and accuracy in implant placement.
Statistical analysis
Trends were assessed using the Cochran-Armitage test for categorical data. The Mann-Kendall test (non-parametric) and linear regression (parametric) were used for continuous data. Generalized linear models were used to control for potential co-variates and to determine the potential independent risk factors for the outcomes of interest. Covariates with a P value <0.2 were entered in our multivariate models. Statistical significance was defined as a P value <0.05. SAS Studio Version 3.4(SAS Institute Inc, Cary, NC, USA) was used for all statistical analyses.
Results
A total of 722 adult patients were included in this study (117 Renaissance, 477 X, 128 Stealth). The mean ± SD CCI was 1.5±1.5, and 54.4% of patients were female. The most common diagnoses included high grade spondylolisthesis (40.6%, N=293), degenerative disc disease (18.4%, N=133), and degenerative scoliosis (17.6%, N=127). Approximately 43.0% (N=309) of cases were performed open (vs. percutaneous), and 10.5% (N=76) of cases had a prior spine surgery. The mean number of instrumented levels was 3.8±3.4 and approximately 20% (N=142) of cases underwent a pelvic fixation. Most patient and operative factors (e.g., gender, smoking status, preoperative diagnosis, total instrumented levels, pelvic fixation, interbody fusion, planned robot screws per patient) were similar across the years (Table 1). The CCI, open vs. percutaneous surgery, prior spine surgery, executed robot screws per patient, and robot type changed significantly over time. Although open surgery (vs. percutaneous) became more common over time, the rate of scoliosis surgery was not significantly different over time (P=0.631). Instead, there was an increase in spinal stenosis (P=0.004) and a decrease in high grade spondylolisthesis (P=0.002) (Table 1).
Table 1
| Variable | All | 2015 | 2016 | 2017 | 2018 | 2019 | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |||||||
| Total No. of patients | 722 | 100 | 40 | 6 | 58 | 8.0 | 165 | 22.9 | 198 | 27.4 | 261 | 36.1 | ||||||
| Female | 393 | 54.4 | 17 | 42.5 | 33 | 56.9 | 92 | 55.8 | 117 | 59.1 | 134 | 51.3 | 0.259* | |||||
| Obese (BMI >30 kg/m2) | 299 | 41.4 | 18 | 45.0 | 18 | 31.0 | 66 | 40.0 | 79 | 39.9 | 118 | 45.2 | 0.198* | |||||
| CCI, mean (standard deviation, SD) | 1.5 (1.5) | 0.95 (1.2) | 1.3 (1.3) | 1.6 (1.5) | 1.5 (1.3) | 1.7 (1.6) | 0.036§ | |||||||||||
| Prior/current smoker | 153 | 21.2 | 3 | 7.5 | 6 | 10.3 | 47 | 28.5 | 45 | 22.7 | 52 | 19.9 | 0.288* | |||||
| Preoperative diagnosis | ||||||||||||||||||
| High grade spondylolisthesis | 293 | 40.6 | 25 | 62.5 | 27 | 46.6 | 71 | 43.0 | 86 | 43.4 | 84 | 32.2 | 0.002* | |||||
| Degenerative disc disease | 133 | 18.4 | 5 | 12.5 | 10 | 17.2 | 31 | 18.8 | 23 | 11.6 | 64 | 24.5 | 0.063* | |||||
| Degenerative scoliosis | 127 | 17.6 | 7 | 17.5 | 10 | 17.2 | 23 | 13.9 | 44 | 22.2 | 43 | 16.5 | 0.631* | |||||
| Spinal stenosis | 116 | 16.1 | 3 | 7.5 | 5 | 8.6 | 24 | 14.5 | 29 | 14.6 | 55 | 21.1 | 0.004* | |||||
| Pseudarthrosis, implant failure | 40 | 5.5 | 0 | 0.0 | 3 | 5.2 | 11 | 6.7 | 12 | 6.1 | 14 | 5.4 | 0.515* | |||||
| Other | 14 | 1.9 | 0 | 0.0 | 3 | 5.2 | 5 | 3.0 | 4 | 2.0 | 2 | 0.8 | 0.146* | |||||
| Operative | ||||||||||||||||||
| Open (vs. percutaneous) | 309 | 42.8 | 5 | 12.5 | 23 | 39.7 | 67 | 40.6 | 101 | 51.0 | 113 | 43.3 | 0.004* | |||||
| Prior spine surgery | 76 | 10.5 | 6 | 15.0 | 12 | 20.7 | 19 | 11.5 | 16 | 8.1 | 23 | 8.8 | 0.020* | |||||
| Total instrumented levels per patient, mean (SD) | 3.8 (3.4) | 3.5 (2.5) | 3.9 (2.8) | 3.4 (3.0) | 4.0 (4.0) | 4.0 (3.4) | 0.407§ | |||||||||||
| Pelvic fixation | 142 | 19.7 | 1 | 2.5 | 18 | 31.0 | 34 | 20.6 | 42 | 21.2 | 47 | 18.0 | 0.902* | |||||
| Interbody fusion | 0.342ǂ | |||||||||||||||||
| TLIF | 122 | 16.9 | 0 | 0.0 | 7 | 12.1 | 32 | 19.4 | 35 | 17.7 | 48 | 18.4 | ||||||
| ALIF | 10 | 1.4 | 0 | 0.0 | 0 | 0.0 | 5 | 3.0 | 3 | 1.5 | 2 | 0.8 | ||||||
| OLIF | 43 | 6.0 | 0 | 0.0 | 3 | 5.2 | 12 | 7.3 | 24 | 12.1 | 4 | 1.5 | ||||||
| XLIF | 19 | 2.6 | 0 | 0.0 | 0 | 0.0 | 9 | 5.5 | 9 | 4.5 | 1 | 0.4 | ||||||
| Planned robot screws per patient, mean (SD) | 7.4 (6.3) | 7.2 (5.5) | 6.8 (4.4) | 6.8 (5.6) | 7.3 (7.1) | 8.0 (6.4) | 0.353§ | |||||||||||
| Executed robot screws per patient, mean (SD) | 6.0 (6.0) | 5.7 (5.0) | 6.0 (4.9) | 6.2 (5.2) | 6.9 (6.5) | 7.9 (6.4) | 0.017§ | |||||||||||
| Robot system | <0.001ǂ | |||||||||||||||||
| Renaissance | 117 | 16.2 | 40 | 100.0 | 58 | 100.0 | 19 | 11.5 | 0 | 0.0 | 0 | 0.0 | ||||||
| X | 477 | 66.1 | 0 | 0.0 | 0 | 0.0 | 146 | 88.5 | 197 | 99.5 | 134 | 51.3 | ||||||
| Stealth | 128 | 17.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 127 | 48.7 | ||||||
*, Cochran-Armitage test; §, Mann-Kendall test (for nonparametric data), and linear regression (for parametric data); ǂ, Chi-square test. CCI, Charlson comorbidity index.
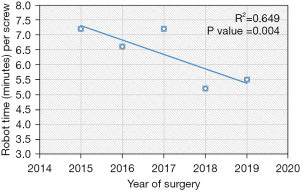
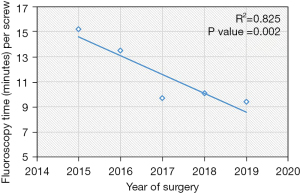
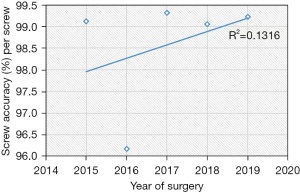
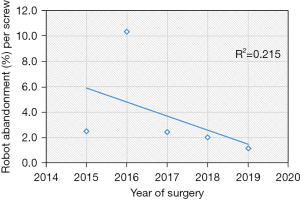
Based on the linear regression trends analyses, the mean robot time per screw decreased from 7.2 to 5.5 minutes (P=0.004) and the mean fluoroscopy time per screw decreased from 15.2 seconds to 9.4 seconds (P=0.002) (Table 2, Figures 1,2). The rate of intraoperative screw exchange for misplaced screw decreased significantly over time (P=0.015, R2=0.1316) (Table 3). There appears to be an outlier in year 2016, but it’s important to note that only the Renaissance robot was used in 2015 to 2016 (Figure 3). Similarly, the robot abandonment reduced significantly over time (P=0.011, R2=0.215) (Table 3). The incidence of non-robot-related intraoperative complications (e.g., dural tear, loss of motor/sensory function, blood transfusion) remained consistently low, but similar between years (Table 3). The overall 90-day reoperation rate was 2.8%, and did not change significantly over the last five years (P=0.828) (Table 4). According to our generalized linear model, both the total instrumented levels and the Renaissance robot were significant predictors for robot abandonment and the decrease in robot time per screw over time was not related to robot type but more related to open vs. percutaneous surgery (estimate: −1.5, 95% confidence interval −2.8 to −0.3, P=0.015) (Table 5). This model controlled for several factors including gender, obesity, CCI, smoker, diagnosis, revision surgery, TIL, pelvic fixation, interbody fusion, and robot type (Table 5, Figure 4). Robot abandonment was most commonly due to registration issues.
Table 2
| Variable | All | 2015 | 2016 | 2017 | 2018 | 2019 | P value |
|---|---|---|---|---|---|---|---|
| Operative time (minutes), mean (SD) | 205 (131.0) | 165 (102.0) | 175 (154.0) | 181 (129.0) | 223 (127.0) | 242 (125.0) | <0.001§ |
| Robot time (minutes), mean (SD) | 41.2 (33.3) | 39.5 (36.1) | 33.2 (24.4) | 35.9 (28.6) | 47.9 (36.2) | 53.5 (39.5) | 0.002§ |
| Robot time per screw (minutes/screw)*, mean (SD) | 6.5 (3.8) | 7.2 (4.8) | 6.6 (4.6) | 7.2 (3.9) | 5.2 (2.3) | 5.5 (2.4) | 0.004§ |
| Total fluoroscopy time (seconds), mean (SD) | 49.0 (36.5) | 63.7 (32.0) | 50.3 (29.5) | 43.3 (34.8) | 47.8 (36.2) | 50.4 (39.5) | 0.034§ |
| Fluoroscopy time per screw (seconds/screw)*, mean (SD) | 10.4 (10.0) | 15.2 (7.9) | 13.5 (10.5) | 9.7 (9.2) | 10.1 (11.0) | 9.4 (9.7) | 0.002§ |
*, Cochran-Armitage test; §, Mann-Kendall test (for nonparametric data), and linear regression (for parametric data).
Table 3
| Variable | All | 2015 | 2016 | 2017 | 2018 | 2019 | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |||||||
| Total No. of executed robot screws | 5,005 | 100.0 | 226 | 4.5 | 340 | 6.8 | 1,018 | 20.3 | 1,367 | 27.3 | 2,054 | 41.0 | ||||||
| Exchange of malpositioned robot screw* | 51 | 1.019 | 2 | 0.9 | 13 | 3.8 | 7 | 0.7 | 13 | 1.0 | 16 | 0.8 | 0.015* | |||||
| Robot abandonment* | 18 | 2.5 | 1 | 2.5 | 6 | 10.3 | 4 | 2.4 | 4 | 2.0 | 3 | 1.1 | 0.011* | |||||
| Due to registration error | 12 | 1.7 | 1 | 2.5 | 6 | 10.3 | 1 | 0.6 | 1 | 0.5 | 3 | 1.1 | 0.008* | |||||
| Due to unreachable anatomy | 4 | 0.6 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 3 | 1.5 | 0 | 0.0 | 0.924* | |||||
| Non-robot complications | ||||||||||||||||||
| Dural tear | 29 | 4.0 | 1 | 2.5 | 1 | 1.7 | 5 | 3.0 | 10 | 5.1 | 12 | 4.6 | 0.217* | |||||
| Loss of motor/sensory function | 5 | 0.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 1.0 | 3 | 1.1 | 0.128* | |||||
| Return to operating room during same index admission | 4 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 2.0 | 0 | 0.0 | 0.740* | |||||
| Perioperative blood transfusion | 67 | 9.3 | 0 | 0.0 | 7 | 12.1 | 21 | 12.7 | 22 | 11.1 | 17 | 6.5 | 0.583* | |||||
| Estimated blood loss (mL), mean [SD] | 255 [407] | 89 [110] | 247 [380] | 239 [362] | 272 [427] | 281 [450] | 0.082§ | |||||||||||
*, Cochran-Armitage test; §, Mann-Kendall test (for nonparametric data), and linear regression (for parametric data).
Table 4
| Variable | All | 2015 | 2016 | 2017 | 2018 | 2019 | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |||||||
| Any reoperation within 90 days after index surgery* | 20 | 2.8 | 1 | 2.5 | 2 | 3.4 | 7 | 4.2 | 1 | 0.5 | 9 | 3.4 | 0.828* | |||||
| Wound complication | 9 | 1.2 | 1 | 2.5 | 0 | 0.0 | 4 | 2.4 | 1 | 0.5 | 3 | 1.1 | 0.519* | |||||
| Neurologic deficit | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0.308* | |||||
| Implant failure | 2 | 0.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.8 | 0.149* | |||||
| Screw malposition | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0.308* | |||||
| Dura fistula | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||||||
| Length of hospital stay, mean (SD)* | 4.1 (2.2) | 5.1 (1.3) | 4.8 (1.8) | 4.1 (2.4) | 3.9 (2.5) | 4.1 (2.0) | 0.007§ | |||||||||||
*, Cochran-Armitage test; §, Mann-Kendall test (for nonparametric data), and linear regression (for parametric data).
Table 5
| Parameter | Estimate | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Robot time per screw* | ||||
| Open vs. percutaneous | −1.5 | −2.8 | −0.3 | 0.015 |
| Fluoroscopic time per screw# | ||||
| X vs. renaissance | −3 | −5 | −1 | 0.003 |
| Stealth vs. renaissance | −7.1 | −9.5 | −4.6 | <0.001 |
| Open vs. percutaneous | −4.9 | −6.7 | −3.2 | <0.001 |
| Robot abandonment& | ||||
| Total instrumented levels | 1.2 | 1.1 | 1.3 | <0.001 |
| X vs. renaissance | −0.9 | −1.3 | −0.51 | <0.001 |
| Length of stay& | ||||
| TIL | 0.32 | 0.24 | 0.4 | <0.001 |
| X vs. renaissance | −0.9 | −1.3 | −0.5 | <0.001 |
| Stealth vs. renaissance | −0.62 | −1.59 | 0.36 | 0.216 |
| Discharge to rehab vs. home | 1.3 | 0.41 | 2.12 | <0.001 |
*, controlling for gender, obesity, CCI, smoker, diagnosis, revision surgery, TIL, pelvic fixation, interbody fusion, and robot type; #, controlling for gender, obesity, CCI, smoker, diagnosis, revision surgery, TIL, pelvic fixation, interbody fusion; &, controlling for gender, obesity, CCI, smoker, diagnosis, open vs. perc., revision surgery, pelvic fixation, interbody fusion. CCI, Charlson comorbidity index.
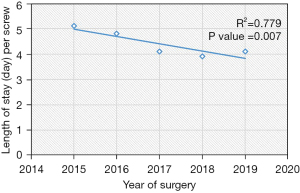
The LOS decreased by nearly 1 day from 2015 to 2019 (P=0.007, R2=0.779) (Table 4, Figure 5), even though the mean CCI index worsened with time (P=0.036) (Table 1). According to our generalized linear model, this decrease in LOS was largely attributed to an increased percentage of patients being discharged to home versus rehab (Table 5). In regards to the decreased mean fluoroscopy time spent per screw, the X vs. Renaissance (estimate: −3.0, 95% confidence interval −5 to −1, P=0.003) and Stealth vs. the Renaissance (estimate: −7.1, 95% confidence interval −9.5 to −4.6), P<0.001) were independent predictors for decreased fluoroscopic time (Table 5). Interestingly, open vs. percutaneous surgery was also associated with reduced radiation time per screw. Other covariates which were not significant in the model included gender, obesity, CCI, smoker, diagnosis, revision surgery, TIL, pelvic fixation, interbody fusion.
Discussion
With the growing number of publications that suggests robots are safe and can achieve comparable outcomes to conventional techniques, much of these publications have limitations such as small patient sizes and single-surgeon or single center series. Furthermore, it is unclear what the impact of robotic technology has made on operative and clinical outcomes over time. This study would be among the first to incorporate a multicenter cohort of patients over 5 years to analyze the trends in patient outcomes and surgical complications after robot assisted spine surgery.
One of the most lauded aspects of robot-assisted spine surgery is the high accuracy and precision of placing pedicle screws, and more recently, S2 alar-iliac screws (10,21-23). In a recent meta-analysis of nine randomized controlled trials, Li et al. demonstrated that robot-assisted techniques were more accurate than the freehand technique (14). In the sub-analysis, the TINAVI robot-assisted technique achieved a higher rate of Grade A screws compared to freehand techniques (relative risk ratio 1.1, 95% confidence interval: 1.06–1.14, P<0.01), while the Renaissance robot-assisted technique showed the same accuracy as the freehand cohorts (relative risk ratio =1.0, 95% confidence interval: 0.96–1.05, P=0.95). Although the Mazor robot is the most extensively studied robot system in literature, there is a paucity of data on more recent Mazor robot systems (10). In a recent case series of 74 screws using the Mazor X, Khan et al. demonstrated excellent screw accuracy (98.7%) (24). In our study, we included the most recent Mazor robot systems and found that the mean screw accuracy was 99%. Furthermore, our linear regression demonstrated that there was a statistically significant trend in improvement in screw accuracy over the last five years. There appears to be an outlier in 2016, but it is important to note that only the Renaissance robot was used in 2015 and 2016 across all surgical centers in this study. Excluding this data point, the screw accuracy remains above 99%. These findings support prior literature on the high accuracy of robot-assisted pedicle screws.
Another important robot complication is robot abandonment, which is less commonly reported in literature. This complication can be multifactorial, including failure to appropriately integrate preoperative CT with intraoperative fluoroscopic images, soft tissue pressure on the guiding arm limiting accurate placement, unreachable anatomy, or drill guide skiving (25-27). According to Macke et al, who performed a retrospective review of pedicle screw placement, registration inaccuracies led to worse pedicle screw accuracy (28). They found that a higher screw accuracy was attained for patients who underwent preoperative CT imaging in the prone position (97.6%) vs. the supine position (92.7%). Interestingly, the low screw accuracy rate noted in 2016 (which only involved the Renaissance robot) corresponded with the high robot abandonment rate in 2016. Nevertheless, our analysis suggests robot abandonment has reduced significantly from 2015 to 2019. According to our generalized linear model, higher total instrumented levels and the use of renaissance were significant risk factors for higher robot abandonment. It is possible that longer constructs involving upper thoracic instrumentation may be more prone to error given the relatively smaller pedicle anatomy in the upper thoracic spine. Furthermore, robot registration of the mid- to upper-thoracic spine relies on a spinous process clamp to connect the robotic arm which may be less stable and thus higher risk for registration failures than when a Schanz pin is used in the posterior superior iliac spine for lower thoracolumbar and pelvic instrumentation. Nevertheless, there was significant improvement in robot reliability over time, which is likely related to the transition from the Renaissance to X after 2016. The specific advancements in technology that likely contribute to the improved screw accuracy and robot abandonment are the newer systems’ ability (Mazor X and Mazor X Stealth) to preoperatively plan out the optimal screw placement based on 3D imaging and therefore better prepare for skive potential based on a patient’s anatomy as well as the serial design of the robot arm, which improves the reach and range of motion for potential implant placement.
A number of prior studies have demonstrated that the operative efficacy or time spent per screw is an area of needed improvement compared to conventional freehand techniques (14,19,29-31). Our study shows that a statistically significant reduction in robot timer per screw was observed over a five-year period. However, our linear model suggests that this was not necessarily due to robot type but more related to the increased rate of using open versus percutaneous surgery. This is not the same as the overall operative time, which includes surgical dissection and closure. In fact, total operative time increased over the years likely due to the increased use of open versus percutaneous surgery. One potential modifiable factor to improve the robot efficiency or robot time spent per screw is overcoming the learning curve required for robotic instrumentation. The learning curve is dependent on a number of factors including surgeon technical ability, teaching, the ease of translating the novel technology in the operating room. Bae et al. studied the learning curve of spinal navigation and found that both screw accuracy and operative time improved considerably after 6 months and plateaued after 12 months (32). In reviewing their most recent procedures, Devito et al. found that their time spent per screw decreased from 13.5 minutes to 10.6 minutes for single-level cases (8). Hu et al. examined the learning curve of using the Renaissance system and found that the success rate of screw placement increased after the first 30 patients (1). In a small case series, Onen et al. experienced a decrease from 15.5 to 5.6 minutes when comparing their initial 13 patients to their subsequent 14 patients (33). Similarly, Hyun et al. found a decrease in time per screw placement from 5.5 to 4.0 minutes over time (17). It is important to note that the actual learning curve will depend on different robot systems, procedures and surgeons.
Fluoroscopic imaging is a potential risk for surgeons, healthcare staff, and the patient due to the ionizing radiation exposure. Studies have shown that fluoroscopy use in other surgical specialties such as interventional cardiology and interventional radiology can increase the risk for skin injury, tissue necrosis, infertility, neoplasia, and cataract formation (34-38). There is good consensus in the literature that robot-assisted cases can reduce the radiation exposure compared to freehand surgeries (29). In comparison to robot-assisted cases, freehand surgeries necessitate fluoroscopic confirmation, which likely increases radiation exposure to both the patient and surgical staff. Hyun et al. performed a prospective randomized control trial and found that freehand surgery was nearly four times than that in the robot-assisted group (17). In a recent cadaveric study, Vaccaro et al. found that significantly fewer fluoroscopic images were needed with robot-assisted cases than freehand techniques (39). In our study, we found that radiation exposure (both total fluoroscopy time and the mean fluoroscopy time per screw) has continued to improve significantly from 2015 to 2019. Our generalized linear model demonstrates that open versus percutaneous surgery, as well as the X and Stealth robots (versus the Renaissance) were significant predictors for decreased fluoroscopic time per screw over the last five years. The reduced radiation exposure may be related to the improved robot registration as well as increased surgeon experience with robotic systems.
There are a number of other limitations that should be considered for this study. First, the cost-effectiveness was not directly addressed in this study. Unfortunately, the current literature on this topic is sparse. Given the associated cost of Mazor X at $550,000, which includes installation and all hardware, it is estimated that ten to twelve lumbar surgeries are needed to pay back this initial cost (7,40). Menger et al. conducted a large, retrospective study on 557 patients and concluded that the application of robotic spine surgery is cost effective, when considering the potential reductions in revision surgery, infections, operative time, and LOS (41). In our study, we demonstrate that operative efficacy has improved and total length of hospital stay has reduced by nearly one day from 2015 to 2019 (P value =0.007, R2=0.779), even though the mean CCI has increased from 0.95 to 1.7. In addition, the 90-day reoperation rates remain consistently low over time. Although the LOS decreased over the years, this was largely attributed to the increased percentage of patients being discharged from home versus rehab and not robot type. In order to validate the possibility that robot-assisted spine surgery can be cost-effective, future work on this topic, specifically analyzing cost parameters, are needed.
Another important aspect of value-based care are patient-reported outcomes, but these metrics were not available at the time of our study. A multicentered comparison of trends with a conventional freehand cohort would be useful, particularly for comparing screw accuracy and time spent per screw by the same surgeons. Unfortunately, this data was not available as most surgeons in this cohort primarily used robot-assisted technology. Another limitation is the lack of granularity in the variable, such as the indication for surgery, which may not always be a single diagnosis and can be a combination of pathologies (e.g., degenerative scoliosis and spinal stenosis). For instance, we observed that there was in increase in open versus percutaneous surgery and expected an increase in deformity cases; instead, there was an increasing trend for lumbar stenosis and spondylolisthesis cases. Next, our study focused on Mazor Renaissance, X, and Stealth systems; therefore, these findings may not be generalizable to other practices which use different robotic systems. Radiation exposure was quantified as fluoroscopy time but radiation dose was not available in our database at the time of our study. Future work should include this and consider comparing preoperative CT-to-fluoroscopy versus scan-and-plan methods and including preoperative radiation (e.g., preoperative CT imaging) to better quantify the total radiation exposure to the patient. Direct measurement of radiation exposure to the surgeon would be another valuable marker to study as well. The learning curve was not directly examined in this study, which may be a major limitation since both 3rd and 4th generation robots were introduced and employed by each site during the study’s time period. Future prospective multicenter studies would be useful in further investigating the impact of surgeon experience on robot-assisted spine surgery outcomes.
Conclusions
Current trends demonstrate that robot screw accuracy, robot abandonment, robot time per screw, and fluoroscopic time per screw have improved significantly over the last five years. This is likely, at least in part, due to the result of increased surgeon experience with robots and the recent advances in robotic technology. The 90-day surgical complication rates remain consistently low and the mean LOS has reduced significantly with time, which may offset the cost of robot-assisted spine surgery; however, future work specifically focusing on cost analyses are needed. Nevertheless, these findings further validate the continued usage of robot-assisted spine surgery and the potential path toward improved value-based care.
Acknowledgments
The abstract was presented at the International Meeting of Advanced Spine Techniques (IMAST) in 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-102/rc
Data Sharing Statement: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-102/dss
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-102/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-102/coif). SZ serves as an unpaid editorial board member of Journal of Spine Surgery. EJ reports consulting fees from Stryker, Medtronic, and Innovasis outside the submitted work. CH reports personal fees from Medtronic, Globus Medical, Spineart, and Innovasis outside the submitted work. CRG reports royalties from Stryker/K2M and Medtronic; consulting fees from Stryker/K2M, Medtronic; educational grant support from Medtronic, is on an advisory board for Stryker/K2M, Medtronic, and Augmedics, and owns stock in Augmedics and NSite. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study, AAAT1470, was approved by the Institutional Review Board of Columbia University Medical Center (No. FWA# 00002636). Written informed consent was exempted because this was a retrospective analysis of de-identified from a multicenter cohort and obtaining informed consent is not required for this type of analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res 2014;472:1839-44. [Crossref] [PubMed]
- Galetta MS, Leider JD, Divi SN, et al. Robotics in spinal surgery. Ann Transl Med 2019;7:S165. [Crossref] [PubMed]
- Alkatout I, Mettler L, Maass N, et al. Robotic surgery in gynecology. J Turk Ger Gynecol Assoc 2016;17:224-32. [Crossref] [PubMed]
- Cole AP, Trinh QD, Sood A, et al. The Rise of Robotic Surgery in the New Millennium. J Urol 2017;197:S213-5. [Crossref] [PubMed]
- Dogangil G, Davies BL, Rodriguez y Baena F. A review of medical robotics for minimally invasive soft tissue surgery. Proc Inst Mech Eng H 2010;224:653-79. [Crossref] [PubMed]
- Vo CD, Jiang B, Azad TD, et al. Robotic Spine Surgery: Current State in Minimally Invasive Surgery. Global Spine J 2020;10:34S-40S. [Crossref] [PubMed]
- D'Souza M, Gendreau J, Feng A, et al. Robotic-Assisted Spine Surgery: History, Efficacy, Cost, And Future Trends. Robot Surg 2019;6:9-23. [Crossref] [PubMed]
- Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine (Phila Pa 1976) 2010;35:2109-15. [Crossref] [PubMed]
- Lieberman IH, Kisinde S, Hesselbacher S. Robotic-Assisted Pedicle Screw Placement During Spine Surgery. JBJS Essent Surg Tech 2020;10:e0020. [Crossref] [PubMed]
- Joseph JR, Smith BW, Liu X, et al. Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg Focus 2017;42:E2. [Crossref] [PubMed]
- Molliqaj G, Schatlo B, Alaid A, et al. Accuracy of robot-guided versus freehand fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery. Neurosurg Focus 2017;42:E14. [Crossref] [PubMed]
- Fiani B, Quadri SA, Ramakrishnan V, et al. Retrospective Review on Accuracy: A Pilot Study of Robotically Guided Thoracolumbar/Sacral Pedicle Screws Versus Fluoroscopy-Guided and Computerized Tomography Stealth-Guided Screws. Cureus 2017;9:e1437. [Crossref] [PubMed]
- Kantelhardt SR, Martinez R, Baerwinkel S, et al. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J 2011;20:860-8. [Crossref] [PubMed]
- Li HM, Zhang RJ, Shen CL. Accuracy of Pedicle Screw Placement and Clinical Outcomes of Robot-assisted Technique Versus Conventional Freehand Technique in Spine Surgery From Nine Randomized Controlled Trials: A Meta-analysis. Spine (Phila Pa 1976) 2020;45:E111-9. [Crossref] [PubMed]
- Fujishiro T, Nakaya Y, Fukumoto S, et al. Accuracy of Pedicle Screw Placement with Robotic Guidance System: A Cadaveric Study. Spine (Phila Pa 1976) 2015;40:1882-9. [Crossref] [PubMed]
- Lonjon N, Chan-Seng E, Costalat V, et al. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J 2016;25:947-55. [Crossref] [PubMed]
- Hyun SJ, Kim KJ, Jahng TA, et al. Minimally Invasive Robotic Versus Open Fluoroscopic-guided Spinal Instrumented Fusions: A Randomized Controlled Trial. Spine (Phila Pa 1976) 2017;42:353-8. [Crossref] [PubMed]
- van Dijk JD, van den Ende RP, Stramigioli S, et al. Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided pedicle screw accuracy. Spine (Phila Pa 1976) 2015;40:E986-91. [Crossref] [PubMed]
- Ghasem A, Sharma A, Greif DN, et al. The Arrival of Robotics in Spine Surgery: A Review of the Literature. Spine (Phila Pa 1976) 2018;43:1670-7. [Crossref] [PubMed]
- Urakov TM, Chang KH, Burks SS, et al. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg Focus 2017;42:E4. [Crossref] [PubMed]
- Shillingford JN, Laratta JL, Park PJ, et al. Human versus Robot: A Propensity-Matched Analysis of the Accuracy of Free Hand versus Robotic Guidance for Placement of S2 Alar-Iliac (S2AI) Screws. Spine (Phila Pa 1976) 2018;43:E1297-304. [Crossref] [PubMed]
- Laratta JL, Shillingford JN, Lombardi JM, et al. Accuracy of S2 Alar-Iliac Screw Placement Under Robotic Guidance. Spine Deform 2018;6:130-6. [Crossref] [PubMed]
- Bederman SS, Hahn P, Colin V, et al. Robotic Guidance for S2-Alar-Iliac Screws in Spinal Deformity Correction. Clin Spine Surg 2017;30:E49-53. [Crossref] [PubMed]
- Khan A, Meyers JE, Siasios I, et al. Next-Generation Robotic Spine Surgery: First Report on Feasibility, Safety, and Learning Curve. Oper Neurosurg (Hagerstown) 2019;17:61-9. [Crossref] [PubMed]
- Barzilay Y, Liebergall M, Fridlander A, et al. Miniature robotic guidance for spine surgery--introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int J Med Robot 2006;2:146-53. [Crossref] [PubMed]
- Kuo KL, Su YF, Wu CH, et al. Assessing the Intraoperative Accuracy of Pedicle Screw Placement by Using a Bone-Mounted Miniature Robot System through Secondary Registration. PLoS One 2016;11:e0153235. [Crossref] [PubMed]
- Schatlo B, Molliqaj G, Cuvinciuc V, et al. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine 2014;20:636-43. [Crossref] [PubMed]
- Macke JJ, Woo R, Varich L. Accuracy of robot-assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg 2016;10:145-50. [Crossref] [PubMed]
- Peng YN, Tsai LC, Hsu HC, et al. Accuracy of robot-assisted versus conventional freehand pedicle screw placement in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Transl Med 2020;8:824. [Crossref] [PubMed]
- Ringel F, Stüer C, Reinke A, et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine (Phila Pa 1976) 2012;37:E496-501. [Crossref] [PubMed]
- Han X, Tian W, Liu Y, et al. Safety and accuracy of robot-assisted versus fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery: a prospective randomized controlled trial. J Neurosurg Spine 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Bai YS, Zhang Y, Chen ZQ, et al. Learning curve of computer-assisted navigation system in spine surgery. Chin Med J (Engl) 2010;123:2989-94. [PubMed]
- Onen MR, Simsek M, Naderi S. Robotic spine surgery: a preliminary report. Turk Neurosurg 2014;24:512-8. [PubMed]
- Kiang SC, Huh AS, Davis JR, et al. Health Care System-Wide Analysis Identifies High Radiation Use Factors and Behaviors in Surgery. Am Surg 2021;87:616-22. [Crossref] [PubMed]
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol 2001;177:13-20. [Crossref] [PubMed]
- Hirshfeld JW Jr, Balter S, Brinker JA, et al. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Circulation 2005;111:511-32. [Crossref] [PubMed]
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol 1994;5:71-84. [Crossref] [PubMed]
- Matityahu A, Duffy RK, Goldhahn S, et al. The Great Unknown-A systematic literature review about risk associated with intraoperative imaging during orthopaedic surgeries. Injury 2017;48:1727-34. [Crossref] [PubMed]
- Vaccaro AR, Harris JA, Hussain MM, et al. Assessment of Surgical Procedural Time, Pedicle Screw Accuracy, and Clinician Radiation Exposure of a Novel Robotic Navigation System Compared With Conventional Open and Percutaneous Freehand Techniques: A Cadaveric Investigation. Global Spine J 2020;10:814-25. [Crossref] [PubMed]
- Fiani B, Quadri SA, Farooqui M, et al. Impact of robot-assisted spine surgery on health care quality and neurosurgical economics: A systemic review. Neurosurg Rev 2020;43:17-25. [Crossref] [PubMed]
- Menger RP, Savardekar AR, Farokhi F, et al. A Cost-Effectiveness Analysis of the Integration of Robotic Spine Technology in Spine Surgery. Neurospine 2018;15:216-24. [Crossref] [PubMed]