
The utility of intraoperative neuromonitoring on simple posterior lumbar fusions—analysis of the National Inpatient Sample
Introduction
Since their introduction in the 1970s, intraoperative neuromonitoring (IOM) techniques such as somatosensory evoked potentials (SSEPs), motor-evoked potentials (MEPs), and electromyography (EMG) have grown to become a routinely used technology in many spine procedures (1). SSEPs are the most commonly used technique, wherein electrodes are used to stimulate peripheral nerves, generating controlled repetitive action potentials that monitor the dorsal column-medial lemniscus pathway (2). MEPs allow for a direct measurement of corticospinal motor tract function while EMGs continuously monitor peripheral nerve roots responsible for specific muscle innervation (2,3). IOM technology is used to potentially detect neurologic injury in real time during these procedures, theoretically reducing the rate of new postoperative neurological deficits.
Several studies have demonstrated the utility of IOM in decreasing the risk of neurologic injury in spinal deformity procedures, likely contributing to the overall increase in IOM usage over the years. However, the routine use of IOM in elective posterolateral lumbar fusions (PLFs) remains controversial (4-9). A number of studies analyzing IOM use in PLF procedures have demonstrated little benefit in reducing postoperative complications (3,10-12). Moreover, several other studies have shown IOM use in spine procedures leads to increased hospitalization cost and procedural time without any change in the rate of neurologic injury (5,11).
The authors queried the National Inpatient Sample (NIS) data set for all patients who underwent a first time elective instrumented PLF for degenerative pathology between 2012 and 2015 in order to gain a better understanding of the efficacy of IOM in the prevention of neurological complications in elective PLF as well as how its use relates to the hospitalization cost and length of stay.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-679).
Methods
Data source
The NIS was analyzed for the years 2012–2015 to identify patients undergoing elective PLF. The NIS is the largest national health database of its kind, consisting of a 20% stratified random sample of all non-federal US hospital discharges. The database contains patient demographics, hospital information, and ICD-9 diagnosis and procedure codes billed for a single hospitalization. The years 2012–2015 were chosen because the database was redesigned in 2012, making analysis of the preceding time period difficult to combine with post-redesign data, and 2015 was chosen as an endpoint because starting in the third quarter of the year, the database was converted from ICD-9 to ICD-10 codes, which also complicates analysis.
Data selection and inclusion/exclusion criteria
Discharges with ICD-9 procedure codes for posterior lumbar fusion (81.07 and 81.08) were identified for patients ≥18 years or older. Patients undergoing thoracolumbar fusions, anterior lumbar fusions, fusions for spinal deformity, and revision fusions were intentionally not included so that the population of interest consisted of routine index lumbar fusions for degenerative spine disease. For similar reasoning, also excluded were discharges with diagnoses of spinal tumors, infections, or trauma. Included patients were then divided into those with and without an ICD-9 procedure code for neuromonitoring (ICD-9 CM 00.94, Table 1). Patient demographics consisting of age, sex, race, primary payer, income quartile of patient ZIP code, and medical comorbidities were extracted along with length of stay, cost of hospitalization, development of postoperative neurological complications, and hospital factors such as teaching status, geographic region, urban or rural location, and hospital bed size. Comorbidities were identified and analyzed using the Elixhauser classification of comorbidities which were analyzed on the basis of count (0, 1, 2, or ≥3). Neurological complication was a binary variable based on the presence or absence of any of the ICD-9 codes 997.00, 997.01, 997.02, 997.09 for “neurologic complications resulting from any services or procedures” (Table 2). Only neurological complications were studied, as opposed to other surgical (e.g., wound infection) and medical [deep vein thrombosis (DVT), pulmonary embolism (PE)] perioperative complications. The three primary outcomes analyzed in the monitoring and non-monitoring patient sub-populations were length of stay, total charge of hospitalization, and development of neurological complications. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). No IRB approval was necessary given the de-identified nature of this national database. No informed consent was obtained as it did not apply to the study.
Full table

Full table
Statistical analysis
Statistical analysis accounted for the complex NIS sample design through the use of appropriate stratification, clustering, and discharge weighting. Missing data was analyzed and imputed using a Markov Chain Monte Carlo method. Age was converted into a categorical variable for three age groups (age 18–40, 41–60, and >60). Number of comorbidities was coded as 0, 1, 2, or ≥3 comorbidities per discharge. Differences in proportions between groups were analyzed with χ2, and differences in means were analyzed with Student’s t-tests. Generalized linear mixed models with hospital as a random intercept using SAS PROC GLIMMIX were used to model length of stay, total charges, and development of complications using Poisson, lognormal, and binary distributions, respectively. Variables included in these statistical models included sex, payer, race, hospital type, hospital geographic region, income quartile by ZIP code, age, hospital bed size, number of comorbidities, year, length of stay, and presence or absence of intraoperative monitoring, in order to adjust for any potential confounding effects of these variables.
Results
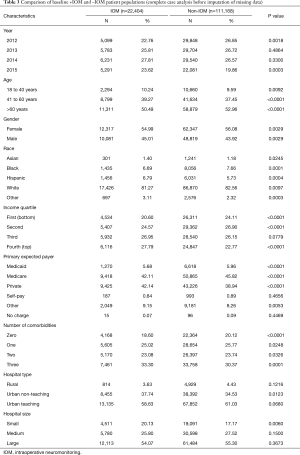
The demographic characteristics of all patients with and without IOM utilization are shown in Table 3. Between the years 2012 and 2015, 22,404 patients were identified in the database that received IOM during their operation. In this same time period, 111,173 patients were identified that underwent a PLF without IOM. The above populations were further broken down into age, gender, race, income percentile, the primary expected payer, number of reported comorbidities, hospital type, and hospital size (Table 3). Our study demonstrated that 27.79% of the IOM cohort were in the top income quartile and 20.60% were in the bottom quartile, in comparison to 22.77% and 24.11% in the group without IOM respectively (P<0.0001). In the IOM group, 42.14% were privately insured in comparison to those that were primarily Medicare (42.11%) and Medicaid (5.68%) covered (P<0.0001). In the group without IOM however, 38.94% were primarily privately insured in comparison to those covered through Medicare (45.82%) or Medicaid (5.96%) (P<0.0001).
Full table
IOM use in elective PLFs was found to have increased from 14.6% in the year 2012 to 19.3% in 2015, which is an overall increase of 1.2% per year (Figure 1). The total charge in hospitalization cost was also found for all patients who received IOM to have increased from $129,384.72 in 2012 to $146,427.79 in 2015 (Figure 2). Cost of stay of the non-IOM patients was $108,503.52 in 2012 and $121,898.88 in 2015. Overall, the total charge of hospitalization was 11% greater in the IOM group when compared to those patients that did not have IOM (95% CI: 11–12%, P<0.001, Figure 2, Table 4). Changes in length of stay over the study period did not demonstrate any identifiable significant trend (Figure 3, Table 4).

Full table
The complication rate for those patients who received IOM during their PLF are shown in Figure 4. IOM did not have a statistically significant impact on the likelihood of developing a neurological complication when adjusting for potential confounders (Figure 4, Table 4).
Discussion
The widespread increase in the use of IOM for spine surgery over the last decade may be partially a result of several studies which have demonstrated efficacy of IOM use in the prevention of new neurological deficits after spinal deformity operations in particular (4,8). In a retrospective study of 3,436 monitored pediatric spinal deformity procedures over 23 years, for example, Thuet et al. concluded that IOM use reduced the incidence of permanent post-operative neurological deficit to only 6 patients (0.17%) while accurately predicting permanent neurologic status in 99.6% of patients (9). In a study analyzing 108,419 cases from the Scoliosis Research Society morbidity and mortality database in which 65% received IOM during surgery, neuromonitoring changes were observed in 11% of patients developing post-operative nerve root deficits, 8% developing cauda equina deficits, and 40% with spinal cord deficits (7). They ultimately found that combined SSEP and MEP use had a sensitivity of 0.43 but a specificity of 0.98 for the detection of neurologic injury (7). Feng et al. similarly reported a sensitivity and specificity of combined MEPs and SSEPs of 92.9% and 99.4%, respectively, in detecting neurologic injury in 175 patients undergoing spinal deformity correction (6).
While there may be some benefit for IOM use in more complex deformity cases, there remains no established consensus on routine IOM use in lower risk elective spine surgery, and evidence in the literature demonstrating a clear, objective benefit for IOM use in instrumented PLF in particular is lacking (4,12-15). Cole et al., for example, retrospectively evaluated the outcomes of 85,640 patients who underwent anterior cervical discectomy and fusion (ACDF), lumbar discectomy, lumbar laminectomy, or lumbar fusion, of which 10,842 patients had IOM. They found that IOM use did not correlate with a reduction in neurologic complications (16). Ajiboye et al. demonstrated a postoperative neurological injury rate of 1.34% without EMG monitoring and 1.36% with EMG monitoring in patients undergoing PLFs, suggesting that routine EMG use may not decrease risk of neurological complications in these procedures (3). Similarly, Alemo et al. evaluated the efficacy of pedicle probe EMG stimulation in 86 patients who underwent placement of 414 lumbar pedicle screws (10). Although pedicle probe EMG stimulation suggested possible neurological compromise in 28 (6.7%) of screws in this series, resulting in 21 being removed and redirected. There were 4 false positives confirmed through direct visualization of the pedicle and nerve root intra-operatively and three false negatives wherein a new neurologic deficit and abnormal CT scan were seen postoperatively in the absence of any indication of nerve root compromise on EMG intraoperatively (10). In a retrospective study by Ajiboye et al., IOM was used in 2,627 out of 15,395 patients who underwent an ACDF with no significant difference in the rate of neurologic injury between groups (0.23% vs. 0.27%) (4). A more recent study analyzing the NIS data set for IOM use in a large group of patients undergoing ACDF similarly showed no significant association between the use of IOM and the development of neurological complications (17).
Another important factor to consider when deciding whether to utilize IOM is cost. In our analysis, an 11% increase in the total charge of hospitalization was detected in the IOM group in comparison to those who did not receive IOM (P<0.001). Additionally, we found that the average length of stay decreased by 0.04 days in the IOM group (P<0.001), although this difference is not clinically significant. Similar results were reported in a study that analyzed 112 patients who underwent a minimally invasive surgery (MIS) transforaminal lumbar interbody fusion (TLIF) at a single institution, 73 of which underwent the procedure with IOM (11). They found that the total surgical cost for patients receiving IOM was significantly higher (P=0.008) by a mean $4,000 (11). They also demonstrated a statistically significant (P=0.009) increase in mean surgical time in the IOM group when compared to those that did not receive IOM (262 vs. 212.46 minutes, respectively) (11). The NIS does not include data on procedural time, and thus this factor could not be included in our analysis.
The results of this study indicate that the use of IOM for PLFs is steadily increasing. Between the years of 2012 and 2015, we found that there was an increase in the utilization of IOM from 14.6% to 19.3%. Interestingly, we found that 27.79% of the patients that received IOM were in the top income quartile and 20.60% were in the bottom quartile, in comparison to 22.77% and 24.11% in the group without IOM respectively (P<0.0001). In the IOM group, 42.14% were privately insured in comparison to those that were primarily Medicare (42.11%) and Medicaid (5.68%) covered (P<0.0001). In the group without IOM however, 38.94% were primarily privately insured in comparison to those covered through Medicare (45.82%) or Medicaid (5.96%) (P<0.0001). Laratta et al. similarly utilized the NIS dataset to assess overall IOM use between the years of 2008 and 2014. They found that IOM use increased by 296% in this time period, with a utilization rate of 45% in privately insured patients when compared to Medicare (36.8%) or Medicaid (9.2%) patients (8). Ajiboye et al. retrospectively queried the PearlDiver Database to evaluate IOM use in scoliosis surgeries between the years of 2005 and 2011, demonstrating a similar increase in overall IOM use from 27% to 46.9% over this time period (18).
These findings challenge the value of IOM in patients undergoing PLFs. The studies discussed above suggest that there may not be any clear benefit to IOM in this scenario and is associated with increased cost and procedural time for patients. A formal cost-benefit analysis of IOM use in spine procedures may be an avenue for further study.
While the choice to use IOM in a procedure is often based partially on surgeon preference or training, the choice may have medicolegal implications as well. IOM records are a part of the medical record that should accurately reflect the patient’s medical history, surgical history, and demographic data as well as being as thorough as possible (19). Additionally, any IOM changes or events that are concerning for potential neurologic injury should be recorded along with any concurrent anesthesia changes or procedural events (20). A log should also be kept detailing the communication of IOM changes to the surgeon at the time of detection (20). Brook et al. reported on the litigation aspects of IOM for neurosurgical procedures, with court case examples from several spine procedures. They state that IOM can potentially be used to support a ruling of direct liability to a surgeon, technologist, or even anesthesiologist, while at other times IOM records can be exculpatory (21). They anticipate that IOM may soon be considered standard of care in the courtroom but, as of now, there are no specific standards for deciding if the misuse or nonuse of IOM technology constitutes as a deviation from the standard (21).
Limitations
A number of limitations are inherent within the use of a large database such as the NIS. The NIS data is pooled from several hospitals nationwide, with varying hospital structures and surgeon practice patterns. Specific clinical information, including the severity of individual patient pathologies, intraoperative events, or the skill of both IOM teams and surgeons themselves, cannot be ascertained. Additionally, the NIS database uses ICD-9 coding systems, which leaves the records subject to inaccuracies in billing, under or overreporting of procedures, and errors in data reporting. One must rely on the accuracy of a given hospital’s coders, who may misclassify diagnoses and procedures. Complications were identified using ICD-9 diagnosis codes for these complications, which may not have been billed consistently between hospitals included in the NIS. In reviewing the financial analysis pertaining to this study, it should be noted that hospital charges do not necessarily reflect payments received by the hospital and this discrepancy was not analyzed in this study. Finally, the single ICD-9 diagnosis code for the use of IOM does not distinguish between the different modalities of neuromonitoring, which would be interesting to analyze independently.
Conclusions
The results of this analysis call into question the routine use of IOM for simple posterior index lumbar fusions for degenerative spine disease. Over 4 years of nationwide hospital data, IOM was associated with an 11% increase in total hospital charges without a statistically significant reduction in complication rate or a clinically significant reduction in length of stay. These relationships hold true when adjusting for patient demographics, hospital factors, year of surgery, and comorbidities. While there may conceivably be benefits to the use of this technology in complex revision fusions or pathologies such as spinal tumors or trauma, we found no meaningful benefit of its application to single-level index posterior lumbar fusions for degenerative spine disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-679
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-679). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). No IRB approval was necessary given the de-identified nature of this national database. No informed consent was obtained as it did not apply to the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tamaki T, Kubota S. History of the development of intraoperative spinal cord monitoring. Eur Spine J 2007;16:S140-6. [Crossref] [PubMed]
- Park JH, Hyun SJ. Intraoperative neurophysiological monitoring in spinal surgery. World J Clin Cases 2015;3:765-73. [Crossref] [PubMed]
- Ajiboye RM, Zoller SD, D'Oro A, et al. Utility of intraoperative neuromonitoring for lumbar pedicle screw placement is questionable: a review of 9957 cases. Spine (Phila Pa 1976) 2017;42:1006-10. [Crossref] [PubMed]
- Ajiboye RM, D'Oro A, Ashana AO, et al. Routine use of intraoperative neuromonitoring during ACDFs for the treatment of spondylotic myelopathy and radiculopathy is questionable: a review of 15,395 cases. Spine (Phila Pa 1976) 2017;42:14-9. [Crossref] [PubMed]
- Ney JP, van der Goes DN, Nuwer MR. Does intraoperative neurophysiologic monitoring matter in noncomplex spine surgeries? Neurology 2015;85:2151-8. [Crossref] [PubMed]
- Feng B, Qiu G, Shen J, et al. Impact of multimodal intraoperative monitoring during surgery for spine deformity and potential risk factors for neurological monitoring changes. J Spinal Disord Tech 2012;25:E108-14. [Crossref] [PubMed]
- Hamilton DK, Smith JS, Sansur CA, et al. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine (Phila Pa 1976) 2011;36:1218-28. [Crossref] [PubMed]
- Laratta JL, Shillingford JN, Ha A, et al. Utilization of intraoperative neuromonitoring throughout the United States over a recent decade: an analysis of the nationwide inpatient sample. J Spine Surg 2018;4:211-9. [Crossref] [PubMed]
- Thuet ED, Winscher JC, Padberg AM, et al. Validity and reliability of intraoperative monitoring in pediatric spinal deformity surgery: a 23-year experience of 3436 surgical cases. Spine (Phila Pa 1976) 2010;35:1880-6. [Crossref] [PubMed]
- Alemo S, Sayadipour A. Role of intraoperative neurophysiologic monitoring in lumbosacral spine fusion and instrumentation: a retrospective study. World Neurosurg 2010;73:72-6; discussion e7. [Crossref] [PubMed]
- Garces J, Berry JF, Valle-Giler EP, et al. Intraoperative neurophysiological monitoring for minimally invasive 1- and 2-level transforaminal lumbar interbody fusion: does it improve patient outcome? Ochsner J 2014;14:57-61. [PubMed]
- Gundanna M, Eskenazi M, Bendo J, et al. Somatosensory evoked potential monitoring of lumbar pedicle screw placement for in situ posterior spinal fusion. Spine J 2003;3:370-6. [Crossref] [PubMed]
- Fehlings MG, Brodke DS, Norvell DC, et al. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976) 2010;35:S37-46. [Crossref] [PubMed]
- James WS, Rughani AI, Dumont TM. A socioeconomic analysis of intraoperative neurophysiological monitoring during spine surgery: national use, regional variation, and patient outcomes. Neurosurg Focus 2014;37:E10 [Crossref] [PubMed]
- Sharan A, Groff MW, Dailey AT, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine 2014;21:102-5. [Crossref] [PubMed]
- Cole T, Veeravagu A, Zhang M, et al. Intraoperative neuromonitoring in single-level spinal procedures: a retrospective propensity score-matched analysis in a national longitudinal database. Spine (Phila Pa 1976) 2014;39:1950-9. [Crossref] [PubMed]
- Badhiwala JH, Nassiri F, Witiw CD, et al. Investigating the utility of intraoperative neurophysiological monitoring for anterior cervical discectomy and fusion: analysis of over 140,000 cases from the National (Nationwide) Inpatient Sample data set. J Neurosurg Spine 2019;31:76-86. [Crossref] [PubMed]
- Ajiboye RM, Park HY, Cohen JR, et al. Demographic Trends in the Use of Intraoperative Neuromonitoring for Scoliosis Surgery in the United States. Int J Spine Surg 2018;12:393-8. [Crossref] [PubMed]
- Malhotra NR, Shaffrey CI. Intraoperative electrophysiological monitoring in spine surgery. Spine (Phila Pa 1976) 2010;35:2167-79. [Crossref] [PubMed]
- Padberg AM, Thuet ED. Intraoperative electrophysiologic monitoring: considerations for complex spinal surgery. Neurosurg Clin N Am 2006;17:205-26. v. [Crossref] [PubMed]
- Brooke M, Irle K. Litigating intraoperative neuromonitoring (IOM). Univ Baltimore Law Rev 2016;45:443-82.






