
Drivers of in-hospital opioid consumption in patients undergoing lumbar fusion surgery
Introduction
It is obvious that the United States is amidst an opioid crisis. This epidemic of opioid use, misuse, and abuse had become a critical public health issue. Americans, comprising only 4.6% of the world’s population, consume 80% of the global opioid supply (1). More than 2 million people are addicted to prescription opioids (2). Drug overdose is the leading cause of accidental death in the US, with more than 20,000 overdose death related to prescription pain relievers in 2015 (3). To help address this desperate public health issue, surgeons must make their best effort to decrease their role in this epidemic, as surgery is associated with an increased risk of chronic postoperative opioid use (4-7).
Prevalence of opioid dependence after spine surgery is high. As much as 38% of patients undergoing major spine surgery are still on opioids one year after surgery (8). A higher rate of preoperative opioid use in patients with spinal diseases may contribute to postoperative opioid dependence (8-10). However, even patients without preoperative opioid use have an increased risk of subsequent chronic opioid dependence in the postoperative period (4,5). The later statistic is what should drive us as surgeons to be educated and thoughtful in how we administer opioids to our patients.
Identifying the drivers of in-hospital opioid consumption can be useful to potentially decrease the incidence of postoperative opioid dependence as in-hospital opioid administration can be a modifiable factor. The aim of this study was to identify the drivers of in-hospital opioid consumption in patients undergoing 1–2-level instrumented lumbar fusions.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-626).
Methods
This is a retrospective cohort study at a single institution. Electronic Medical Record analysis identified consecutive patients who underwent a one- or two-level instrumented lumbar fusions for degenerative lumbar conditions from 2016 to 2018 using the hospital administrative database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of University of Louisville/Norton Healthcare (# 18.1197) and individual consent for this retrospective analysis was waived.
Postoperative opioids were administered by nurses based on doctors’ PRN order and pain severity. In-hospital, total daily opioid consumption, including oral, intravenous, or transdermal administration was calculated and converted to the morphine milligram equivalents (MMEs) using MME conversion factors (Table 1). In brief, MMEs of each opioid was calculated based on the following formula.
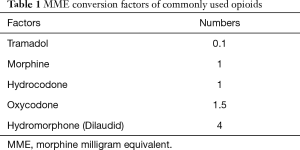
Full table
[1]
Then, MMEs of used opioids were added.
Linear regression analysis was used to determine associations between postoperative day (POD) cumulative in-hospital MMEs and the patients’ baseline characteristics including body mass index (BMI), race, American Society of Anesthesiologists (ASA) grade, smoking status, marital status, preoperative daily opioid use, insurance type, zip code, number of fused levels, approach and preoperative opioid use. Preoperative opioid use including type of opioid, dose and frequency, was asked before surgery. Zip codes were classified into medically “underserved” or not based on government designation; areas with a high prevalence of health conditions in combination with a higher than average poverty rate and less access to healthcare, were designated as “underserved”. Cumulative MME’s up to POD 4 were used as the majority patients were discharged by POD 5.
Statistical analysis
A multivariate linear regression analysis was performed to identify risk factors for in-hospital opioid consumption. All statistical analyses were performed using SPSS Statistics 25 (IBM Corp., Armonk, NY). A statistical significance was defined as P value <0.05.
Results
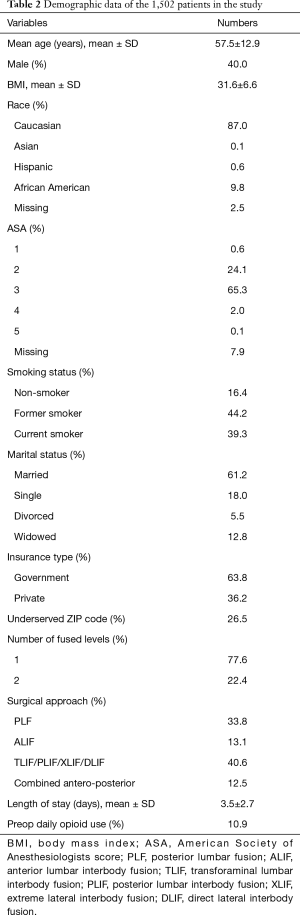
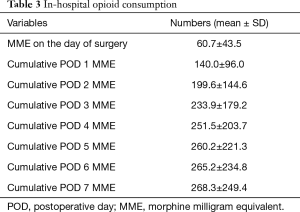
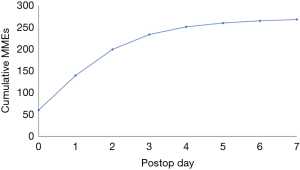
A total of 1,502 patients, 601 (40%) male, mean age of 57.5 years, were included. Patients’ demographic data are shown in Table 2. The mean BMI was 31.6, and patients were predominantly Caucasian. 39.3% were current smokers, while former smokers comprised 44.2%; 26.5% lived in underserved zip codes. The majority of patients (77.6%) underwent single level fusion. Anterior lumbar interbody fusion (ALIF) was done in 13.1% while combined antero-posterior procedure was done in 12.5%. Only 163 (11%) reported active daily opioid use prior to surgery. Total cumulative MMEs are shown in Table 3. Cumulative MMEs reached plateau at POD 4 being 251.5±203.7 (Figure 1).
Full table
Full table
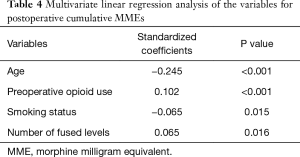
Younger age, preoperative opioid use, current smokers and more levels fused were associated with greater cumulative in-hospital MMEs with the R square of 0.079 (Table 4). There were no associations with surgical approach, zip code, ASA grade, marital status, BMI, race or insurance type.
Full table
Discussion
Amid the current opioid epidemic, it is imperative for surgeons to identify high risk patients preoperatively, as part of an effort to minimize chronic opioid use after surgery. Additionally, it would be wise from our specialty perspective, to take ownership of, or at minimum to reduce surgeon contribution to this atrocious public health problem. A number of risk factors for postoperative chronic opioid use have been reported, most commonly and obvious is preoperative opioid use, followed by younger age, depression, substance use disorder, preoperative pain conditions, and smoking (8-13). These studies did not focus on in-hospital opioid administration even though it may well be critical, in particular for opioid naïve patients (14,15). Ge et al. showed that the amount of in-hospital opioid consumption following transforaminal lumbar interbody fusion (TLIF) is associated with postoperative chronic opioid dependence in patients with and without preoperative opioid use. Compared with patients receiving 250–500 in-hospital MMEs, those receiving <250 in-hospital MMEs had a 3.73 times lower probability of requiring opioids at 6 months follow-up whereas those receiving >500 in-hospital MMEs had a 4.84 times greater probability of requiring opioids at 6 months (16). This study also demonstrated more than 500 MMEs during admission is a risk factor for postoperative chronic opioid use (16).
Of the four identified factors associated with in-hospital opioid consumption in this study, preoperative opioid use and smoking are potentially modifiable. There are numerous reports examining the association between preoperative opioid use and postoperative chronic opioid dependence (6,8,10-12). A recent systematic review revealed the incidence of persistent postoperative opioid use, whose definition was variable depending on studies (90-day to 1-year postop), ranges from 35% to 77% for patients with preoperative opioid use and from 0.6% to 26% for opioid-naive patients in major surgical procedures (11). In addition, duration of preoperative opioid use was the most important predictor of continued use following lumbar spine surgery (13). Considering these findings, it is optimal to minimize preoperative opioid exposure, and to spend time educating patients about the potential downstream undesired affects. In our cohort, only 11% of patients were using daily opioids preoperatively. However, some studies have reported that up to 72.1% of patients presenting for major spine surgery were chronically using opioids before surgery (8). Preoperative opioid use was still risk factor for postoperative chronic opioid use in this study (8) despite this big discrepancy in the rate of preoperative opioid use might affect the results of statistical analysis. Thus, weaning patients off opioids prior to surgery may decrease in-hospital consumption and subsequently decrease chronic opioid use.
We also identified smoking as another modifiable factor. Multiple studies have showed the association between smoking and postoperative opioid dependence (6,11,17). Smoking has negative impact on all aspects of surgical treatment in spine surgery, including patient-reported outcomes, infection rate, pseudarthrosis, reoperation rate and complications (18-21). Literature supports smoking cessation as an effective tool in mitigating negative outcomes in spine surgery (21). The current study provides another reason for advocating smoking cessation prior to lumbar fusion surgery.
We also identified two risk factors, younger age and more levels fused, that are more difficult to be modified. Debate exists in terms of the impact of age on postoperative opioid dependence. Sharma et al. investigated risk factors associated with opioid dependence in 10,708 patients undergoing surgery for degenerative spondylolisthesis, in which younger age was an independent predictor of opioid dependence following surgery (12). On the other hand, Sun et al. examined risk factors associated with opioid dependence in patients undergoing major surgery, and identified age older than 50 years was associated with chronic opioid use with odds ratio of 1.74 (5). Interestingly, Kalakoti et al. reported that younger patients had a lower likelihood of opioid use following PLIF or TLIF whereas younger age was associated with higher likelihood of opioid use following posterior lumbar fusion (PLF) (9). However, the majority of previous studies did not find an association between age and chronic opioid dependence (8,10). We found only one study focusing on in-hospital opioid consumption (16). This study did not show a difference between in-hospital opioid consumption among patients of different age, but showed patients taking preoperative opioids were significantly younger than opioid naïve patients. Further study is necessary to conclude for the impact of age.
With regards to more levels requiring fusion or surgical invasiveness, a more invasive procedure intuitively may require more opioids. Modern technology allows for more complex spine procedures and spine surgery has become more invasive. Opioids are indispensable in perioperative pain management in spine surgery. Opioids are a necessary component of postoperative pain control, but efforts should be made to limit the amount. To decrease perioperative opioid consumption, multi-modal pain control (MMPC) approach has been developed (22). MMPC uses multiple agents that target several different pathways and mediators involved in nociception to improve analgesic effect (22,23). MMPC has been reported to be associated with less postoperative pain and opioid consumption (24-29). MMPC may be a good option to minimize in-hospital opioid consumption and subsequent chronic opioid dependence. Opioid free anesthesia (OFA) techniques have been developed which even further reduces in hospital exposure to opioids (30). Whether it is exposure, dosage, or duration of opioids, in theory they all can contribute to a long-term dependence and if possible, we should try to minimize our opioid consumption.
There are several limitations in this study. First, this is a retrospective study in a single institution, making external validity unclear. Second, we did not evaluate chronic opioid dependence after discharge. Partly because of the inherent flaws in studying out of hospital opioid consumption. Typically, this is self-reported and the validity of actual usage versus other alternatives cannot be verified. Also, preoperative opioid use was based on patients’ report and might have recall bias. In turn, in-hospital consumption is monitored strictly. Further studies are warranted to see the impact of in-hospital opioid consumption on the transition to chronic opioid dependence (14,15).
In conclusion, use of opioids prior to surgery and smoking are modifiable risk factors for higher in-hospital opioid consumption and can be targets for intervention prior to surgery in order to decrease in-hospital opioid use. Additionally, although levels to be fused or surgical invasiveness and age are not necessarily modifiable, they are still identifiable risk factors. When counselling patients on appropriate expectations prior to undergoing lumbar fusion surgery, it potentially beneficial to identify high-risk patients and counsel them on opioid consumption.
Acknowledgments
This study was partly presented as a podium in the annual meeting of the North American Spine Society in 2019.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-626
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-626
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-626). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of University of Louisville/Norton Healthcare (# 18.1197) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008;11:S63-88. [PubMed]
- Murthy VH. Ending the Opioid Epidemic - A Call to Action. N Engl J Med 2016;375:2413-5. [Crossref] [PubMed]
- Rudd RA, Seth P, David F, et al. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep 2016;65:1445-52. [Crossref] [PubMed]
- Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. Bmj 2014;348:g1251. [Crossref] [PubMed]
- Sun EC, Darnall BD, Baker LC, et al. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 2016;176:1286-93. [Crossref] [PubMed]
- Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 2017;152:e170504. [Crossref] [PubMed]
- Hah JM, Bateman BT, Ratliff J, et al. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth Analg 2017;125:1733-40. [Crossref] [PubMed]
- Dunn LK, Yerra S, Fang S, et al. Incidence and Risk Factors for Chronic Postoperative Opioid Use After Major Spine Surgery: A Cross-Sectional Study With Longitudinal Outcome. Anesth Analg 2018;127:247-54. [Crossref] [PubMed]
- Kalakoti P, Hendrickson NR, Bedard NA, et al. Opioid Utilization Following Lumbar Arthrodesis: Trends and Factors Associated With Long-term Use. Spine (Phila Pa 1976) 2018;43:1208-16. [Crossref] [PubMed]
- Hills JM, Pennings JS, Archer KR, et al. Preoperative Opioids and 1-year Patient-reported Outcomes After Spine Surgery. Spine (Phila Pa 1976) 2019;44:887-95. [Crossref] [PubMed]
- Kent ML, Hurley RW, Oderda GM, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative-4 Joint Consensus Statement on Persistent Postoperative Opioid Use: Definition, Incidence, Risk Factors, and Health Care System Initiatives. Anesth Analg 2019;129:543-52. [Crossref] [PubMed]
- Sharma M, Ugiliweneza B, Aljuboori Z, et al. Factors predicting opioid dependence in patients undergoing surgery for degenerative spondylolisthesis: analysis from the MarketScan databases. J Neurosurg Spine 2018;29:271-8. [Crossref] [PubMed]
- Schoenfeld AJ, Belmont PJ Jr, Blucher JA, et al. Sustained Preoperative Opioid Use Is a Predictor of Continued Use Following Spine Surgery. J Bone Joint Surg Am 2018;100:914-21. [Crossref] [PubMed]
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018;360:j5790. [Crossref] [PubMed]
- Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain 2017;18:1374-83. [Crossref] [PubMed]
- Ge DH, Hockley A, Vasquez-Montes D, et al. Total Inpatient Morphine Milligram Equivalents Can Predict Long-term Opioid Use After Transforaminal Lumbar Interbody Fusion. Spine (Phila Pa 1976) 2019;44:1465-70. [Crossref] [PubMed]
- Qureshi R, Werner B, Puvanesarajah V, et al. Factors Affecting Long-Term Postoperative Narcotic Use in Discectomy Patients. World Neurosurg 2018;112:e640-4. [Crossref] [PubMed]
- How NE, Street JT, Dvorak MF, et al. Pseudarthrosis in adult and pediatric spinal deformity surgery: a systematic review of the literature and meta-analysis of incidence, characteristics, and risk factors. Neurosurg Rev 2019;42:319-36. [Crossref] [PubMed]
- Tetreault L, Palubiski LM, Kryshtalskyj M, et al. Significant Predictors of Outcome Following Surgery for the Treatment of Degenerative Cervical Myelopathy: A Systematic Review of the Literature. Neurosurg Clin N Am 2018;29:115-27.e35. [Crossref] [PubMed]
- Blood AG, Sandoval MF, Burger E, et al. Risk and Protective Factors Associated with Surgical Infections among Spine Patients. Surg Infect (Larchmt) 2017;18:234-49. [Crossref] [PubMed]
- Jackson KL 2nd, Devine JG. The Effects of Smoking and Smoking Cessation on Spine Surgery: A Systematic Review of the Literature. Global Spine J 2016;6:695-701. [Crossref] [PubMed]
- Beverly A, Kaye AD, Ljungqvist O, et al. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol Clin 2017;35:e115-43. [Crossref] [PubMed]
- Gritsenko K, Khelemsky Y, Kaye AD, et al. Multimodal therapy in perioperative analgesia. Best Pract Res Clin Anaesthesiol 2014;28:59-79. [Crossref] [PubMed]
- Crisologo PA, Monson EK, Atway SA. Gabapentin as an Adjunct to Standard Postoperative Pain Management Protocol in Lower Extremity Surgery. J Foot Ankle Surg 2018;57:781-4. [Crossref] [PubMed]
- Merchea A, Lovely JK, Jacob AK, et al. Efficacy and Outcomes of Intrathecal Analgesia as Part of an Enhanced Recovery Pathway in Colon and Rectal Surgical Patients. Surg Res Pract 2018;2018:8174579. [Crossref] [PubMed]
- Talboys R, Mak M, Modi N, et al. Enhanced recovery programme reduces opiate consumption in hip hemiarthroplasty. Eur J Orthop Surg Traumatol 2016;26:177-81. [Crossref] [PubMed]
- Niemelainen M, Kalliovalkama J, Aho AJ, et al. Single periarticular local infiltration analgesia reduces opiate consumption until 48 hours after total knee arthroplasty. A randomized placebo-controlled trial involving 56 patients. Acta Orthop 2014;85:614-9. [Crossref] [PubMed]
- Dwyer AJ, Thomas W, Humphry S, et al. Enhanced recovery programme for total knee replacement to reduce the length of hospital stay. J Orthop Surg (Hong Kong) 2014;22:150-4. [Crossref] [PubMed]
- Machin JT, Phillips S, Parker M, et al. Patient satisfaction with the use of an enhanced recovery programme for primary arthroplasty. Ann R Coll Surg Engl 2013;95:577-81. [Crossref] [PubMed]
- Beloeil H. Opioid-free anesthesia. Best Pract Res Clin Anaesthesiol 2019;33:353-60. [Crossref] [PubMed]


