
Navigation-assisted full-endoscopic spine surgery: a technical note
Introduction
Full-endoscopic spine surgery (FESS) is a less invasive technique compared with the conventional open or mini-open technique. Several studies have demonstrated the efficacy of FESS (1-4). Intraoperative X-ray fluoroscopic guidance is typically used during FESS for the confirmation of orientation. However, X-ray fluoroscopy provides only intermittent two-dimensional (2D) information. Conversely, three-dimensional (3D) navigation using cone beam computed tomography (CBCT) or computed tomography (CT) can provide imaging projections of the operative field and instruments in three dimensions (5,6). We herein report the adaptation of 3D navigation during FESS in a hybrid operating room (OR) which allows for the integration of intraoperative CBCT data into the navigation system to renew the registered information. This is the first report about the adaptation of the real-time 3D navigation during FESS in a hybrid OR. The feasibility and usefulness of this system have been discussed.
Methods
In total, 23 patients who underwent FESS accompanied by 3D navigation in a hybrid OR between January 2016 and November 2019 were enrolled. Medical records, intraoperative endoscopic video, navigation records, and navigation video were retrospectively reviewed to evaluate the feasibility and usefulness of 3D navigation in FESS.
Preoperative setting
The surgical setup employed is demonstrated in Figure 1A,B. A multiaxis robotic C arm (Artis zeego; Siemens AG, Forchheim, Germany) and image-guided navigation system (BrainLAB Curve; Brainlab, Munich, Germany) were available in this hybrid OR. After loading the preoperative thin-slice (2-mm) CT scan into the navigation system, the camera of the navigator was placed to direct toward the reference arc, which was placed in the pole attached with the operating table for patients with thoracolumbar disease and in the Mayfield skull clamp for patients with cervical spine disease (Figure 1C,D,E,F). For patients with lumbar pathology, the navigation reference array was always placed within 40 cm from the region of interest. Automatic registration was used for all cases in this hybrid OR.

CBCT, image fusion and automatic registration
After positioning the patient and before starting surgery, 3D CT images were obtained using CBCT and transferred to the computer-assisted navigation system. The imaging reconstructions were generated in a few seconds. The resulting images were automatically registered to the patient, because the C-arm was previously calibrated and is tacked using reflective stickers. The intraoperative CT data was integrated with the preoperatively registered 3D simulation data.
Operative technique
All navigated probes and instrumentation were calibrated to check the accuracy. Navigation was used both as confirmation of position and to access direction. For the transforaminal approach, the 3D navigation system can provide projection images directed by the navigation probe from the skin to the target or the foramen by estimating the Kambin’s triangle. For the interlaminar approach, a paramedian skin incision was planned using a navigated probe. After setting a working cannula, we can use the navigated spatula through the working channel.
Case presentation
Case 1
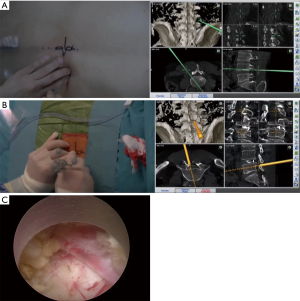
A 54-year-old man with intermittent claudication since >3 years was diagnosed with lumbar canal stenosis due to L4–L5 disc herniation and thickened ligamentum flavum. He was suffering from the right leg pain. The patient underwent discography via the transforaminal approach and percutaneous endoscopic lumbar discectomy via the interlaminar approach. The surgery was performed in the hybrid OR guided by real-time 3D navigation. After the patient was positioned on the table, CBCT was performed before the start of surgery; the CT data was registered into the navigation system when the reference arc was placed in the pole on the operating table. Before the insertion of the working cannula mounting endoscope, intraoperative discography was performed for diagnosis of the condition and to determine the extent of the nucleus; this was also added as the renewed CT data to the navigation system. Next, 3D navigation was used to determine the trajectory via the transforaminal route, which provided the visualized 3D information, or the patient’s images and projection images directed by the navigation probe (Figure 2A). The working cannula was inserted under guidance by 3D navigation (Figure 2B). After confirmation of the surrounding structures, discectomy and decompression were completely achieved endoscopically (Figure 2C). During the entire procedure, 3D navigation allowed real-time visualization of the position. The postoperative course was uneventful, and the patient’s gait disturbance and right leg pain subsided immediately.
Case 2
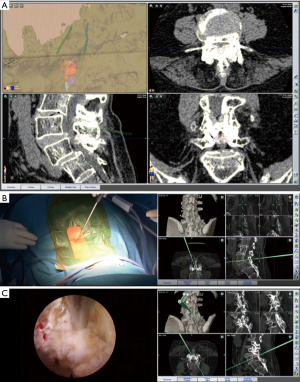
The patient was a 74-year-old woman with a history of persistent low back pain and intermittent claudication for 5 years. She developed numbness and motor weakness in both legs 3 months prior to referral to our clinic. Radiographic examination showed grade 1 degenerative spondylolisthesis. Preoperative magnetic resonance imaging (MRI) revealed a decrease in the disc height at the level of L4–L5 and L4–L5 anterolisthesis. Reconstructed CT images showed severe narrowing of the interlaminar space due to the degenerative changes (Figure 3A). Owing to her comorbidity, she underwent less invasive percutaneous endoscopic lumbar laminectomy. The patient was placed in the prone position on the operative table, and then, CBCT was performed. CBCT data was registered as current data in the navigation system. The real-time 3D navigation system accurately guided the surgeon to the narrowed right interlaminar space at the level of L4–L5 (Figure 3B). The interlaminar space was opened by partial laminectomy, and the thickened flavum ligamentum was removed. We confirmed the entry point to the contralateral side by navigation and direct visualization. Subsequently, we were able to perform decompression of the lateral recess on the contralateral side, protecting the dura matter with flavum ligamentum (Figure 3C). Complete decompression was achieved until the contralateral lateral recess. Postoperatively, the patient showed complete resolution of the symptoms.
Case 3
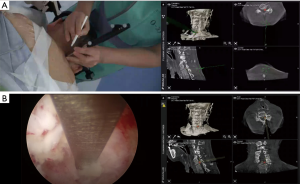
A 64-year-old man presented with left arm pain and numbness for 6 months. On physical examination, the patient showed 4+ motor strength in the left triceps, attenuated brachioradialis reflex on the left side, and numbness in the area of C7 distribution. MRI of the cervical spine demonstrated left foraminal encroachment at the C6–C7 level. Despite conservative treatment including physical therapy and epidural steroid injections, his arm pain aggravated. Treatment options were discussed with the patient, and he opted for the less invasive percutaneous endoscopic posterior foraminotomy. The patient was placed in the prone position, and the head was fixed with a three-point skull clamp in the hybrid OR. After positioning the patient, CBCT was performed; the CT data obtained in the current prone position was registered into the navigation system. The entry point was determined based on the appropriate trajectory alignment visualized on the monitor in the navigation system (Figure 4A). Along the projection image on 3D navigation, the skin incision was made, and the navigated obturator was inserted into the target point. The working channel was inserted through the obturator. We used a rigid endoscope with a working channel through which endoscopic instruments can be used under continuous endoscopic high definition visualization. We were able to confirm the anatomy using the navigated instrument through the working channel. By providing accurate orientation, the 3D navigation system helped identify the lamina, facet, and ligamentum flavum (Figure 4B). After cleaning off the tissue surrounding the foramen, the endoscopic drill was used to perform foraminotomy at the laminar facet junction at C6–C7. Finally, the radiofrequency probe was used to achieve hemostasis. The postoperative course was uneventful. The patient experienced alleviation of pain immediately after surgery.
Results
The present study cohort comprised 17 patients with lumbar spine disease and 6 patients with cervical spine disease (Table 1). None of the patients experienced any complications associated with real-time 3D navigation-assisted FESS. We found a remarkable concordance of the location of intraoperative landmarks between the endoscopic direct vision and the 3D navigation system. The real-time 3D navigation provided accurate guidance in all patients. The median procedure time was 112 minutes. The median scan time was 7 minutes. The median verification time was 4 minutes. All patients in the present series had satisfactory improvement after surgery.

Full table
Discussion
We presented a workflow for real-time 3D navigation-assisted FESS performed in the hybrid OR. The use of X-ray fluoroscopy for intraoperative orientation was not required in any of the patients in this series. The 3D navigation system showed high accuracy for trajectory alignment and intraoperative orientation and was useful for FESS.
Radiation exposure is always a major concern in spine surgery, which is attributable to fluoroscopy (7). However, the use of a navigation guided system entails minimal or no radiation exposure to the surgeons, as surgeons do not need to manipulate any instrument under radiation. Conversely, the use of real-time 3D navigation system is associated with increased radiation exposure to patients compared with that of the conventional procedure using X-ray fluoroscopy because of the need for repeated intraoperative CT scans. However, the effective dose of radiation for the initial registration scan in navigation using CT scan for spine surgery have been reported to be only 3.37±0.93 mSv (range, 1.59–5.01 mSV) (8). From another perspective, the irradiation time during use of X-ray fluoroscopy in spine surgery is quite limited. Therefore, we cannot always use fluoroscopy during surgery. However, the use of navigation system provides real-time information about the position and projection during the entire surgery.
Endoscopic procedure is less invasive, but provides limited visual access to the operative field. Therefore, it is difficult to identify anatomically contiguous structures, especially for inexperienced surgeons. The 3D navigation providing real-time information about orientation helps surgeon in identifying surgical anatomy. Furthermore, the distance from the entry point to the target is also calculated, and the current position of the tip of the probe, needle, and the working cannula are confirmed on the navigation monitor next to the monitor of endoscope. The feasibility of using the 3D navigation system and the comfort level of the surgeon in manipulating instruments depends on the setting of monitors of the endoscope and the navigation system. Carl et al. (8) investigated the registration accuracy of navigation using intraoperative CT and automated patient registration. They reported a target registration error of 0.86±0.38 mm in overall surgery including cranium and 0.80±0.28 mm in spine surgery (8). Registration error of <1 mm for 3D navigation is considered acceptable.
As a limitation of our study, the reference for navigation was not placed in the iliac crest or spinous process in patients with thoracolumbar disease. Bony structure is believed to be better for the site of placement for reference of navigation from the view point of accuracy. But Intraoperative imaging-bases registration does not need patients or user interaction, so it can be automated. This offers the possibility of reduced registration errors. The hybrid operation room and the automatic registration system of the navigation system give us the real-time navigation image which is practically accurate and reliable and enables us to confirm the key anatomic structure from navigation and endoscopic view and perform full endoscopic surgery safely with these minimally invasive methods.
Conclusions
The aim of this technical note is to provide preliminary evidence of feasibility of endoscopic spine surgery assisted by neuronavigation system in hybrid operation room. Our experience is limited and needed to be expand to verify the feasibility and demonstrate the usefulness and contraindications of the procedure. However, these preliminary results in our opinion are encouraging.
Acknowledgments
We would like to express our gratitude to the staff of the radiological engineering team at the Osaka Police Hospital, for being in charge of the operating robotic C arm and obtaining CT scan in the hybrid OR.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-2019-fess-19). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin GX, Kotheeranurak V, Mahatthanatrakul A, et al. Worldwide research productivity in the field of full-endoscopic spine surgery: a bibliometric study. Eur Spine J. 2020;29:153-60. [Crossref] [PubMed]
- Oyelese AA, Fridley J, Choi DB, et al. Minimally invasive direct lateral, retroperitoneal transforaminal approach for large L1-2 disc herniations with intraoperative CT navigational assistance: technical note and report of 3 cases. J Neurosurg Spine 2018;29:46-53. [Crossref] [PubMed]
- Komatsu J, Muta T, Nagura N, et al. Tubular surgery with the assistance of endoscopic surgery via a paramedian or midline approach for lumbar spinal canal stenosis at the L4/5 level. J Orthop Surg (Hong Kong) 2018;26:2309499018782546. [Crossref] [PubMed]
- Wagner R, Telfeian AE, Iprenburg M, et al. Minimally invasive fully endoscopic two-level posterior cervical foraminotomy: technical note. J Spine Surg. 2017;3:238-42. [Crossref] [PubMed]
- Guha D, Moghaddamjou A, Jiwani ZH, et al. Utilization of Spinal Intra-operative Three-dimensional Navigation by Canadian Surgeons and Trainees: A Population-based Time Trend Study. Can J Neurol Sci 2019;46:87-95. [Crossref] [PubMed]
- Murayama Y, Irie K, Saguchi T, et al. Robotic digital subtraction angiography systems within the hybrid operating room. Neurosurgery 2011;68:1427-33. [Crossref] [PubMed]
- Hayda RA, Hsu RY, DePasse JM, et al. Radiation Exposure and Health Risks for Orthopaedic Surgeons. J Am Acad Orthop Surg 2018;26:268-77. [Crossref] [PubMed]
- Carl B, Bopp M, Saß B, et al. Reliable navigation registration in cranial and spine surgery based on intraoperative computed tomography. Neurosurg Focus 2019;47:E11. [Crossref] [PubMed]


