Multilevel cervical arthroplasty—clinical and radiological outcomes
Introduction
Cervical disc arthroplasty (CDA) is increasingly regarded as an alternative to anterior cervical discectomy and fusion (ACDF), traditionally accepted as the gold standard technique to treat single level cervical disc disease (CDD) (1-6). ACDF is also an effective option for multi-level disease with good long-term outcomes (7,8). However, it is associated with significant drawbacks. The most obvious is the elimination of motion at the index levels with a biomechanical impact (9,10), namely increased stress on the adjacent natural level (9), contributing to adjacent segment disease (ASD) rates of up to 25% at 10 years (11). Hardware-related complication rates are also not irrelevant with instrument failure (12), pseudarthrosis incremental to the number of operated levels (12,13), and dysphagia (14). When it comes to 3- or 4-level fusion constructs, the rigidity of the fused spine may impose a significant burden on the patients’ quality of life.
Despite a wide variety of implants with intrinsic biomechanical profiles, CDA has shown good clinical and radiological outcomes, ranging from non-inferior to statically significant superiority when compared to ACDF for single-level CDD (1,15-19). CDA also seems to be more cost-effective than cervical fusion (20,21). However, this does not come without complications, such as high-grade heterotopic ossification (HO), reported being as high as 62% (22,23), and anterior vertebral body bone loss (24).
Multilevel procedures only recently gained momentum with the first FDA approval for 2-level arthroplasty in 2013 (25) followed by other implants and a number of IDE studies under way (25). Hybrid constructs (CDA adjacent to fusion) have also been studied with favorable outcomes (26-28). There are, however, few studies on the outcomes of 3- and 4-level CDA only. Most of these studies focus on old design devices, non-constrained or semi-constrained implants with scarce published literature on third generation implants with 6 degrees of freedom.
We reviewed the multilevel cervical disc cases operated on our department with focus on the clinical outcomes and biokinematics of CDA, the hypothesis being that 2 to 4 level procedures are safe and effective when indicated.
Methods
Patients
Between 2013 and 2018, patients with multilevel CDD were treated with CA, ranging from 2- to 4-level procedures. All patients involved were older than 18, had myelopathy, radiculopathy or combined features, cervical imaging (CT scan and MRI) showing cervical cord compression and/or foramen stenosis compatible with their symptoms with at least 1 year of follow-up. The patients had pre- and post-operative neutral and dynamic X-rays at last follow-up.
Clinical data was collected on their epidemiological characteristics (gender, age at time of surgery, topography and number of operated levels, duration of follow-up). Patients were excluded if they have had previous cervical spine surgery, or were treated with hybrid constructs, or lacked complete clinical and/or radiological data.
The contra-indications for CDA were significant cervical spondylosis, cervical lysthesis, OPLL and facet arthropathy. Low ROM in the pre-operative dynamic study was not an absolute contra-indication per se, if after decompression and uncus release the index level was judged to have appropriate mobility. To this date, no patient previously planned for CDA had to be converted to ACDF due to this issue.
Implant and technique
We used the same type of restrained third generation implant with six degrees of freedom (29) for all patients included in this study. Patients treated for 2 and 3 level disc disease with other implants were excluded from this cohort. All patients were treated by the same senior surgeon (Óscar L. Alves).
A standard anterior cervical approach was undertaken while the patient on supine position. Positioning of head and neck was first assessed under lateral fluoroscopy to avoid excessive extension of the neck, what we believe is linked with later implant subsidence, and then secured with the patient’s chin tapped to the table for stability. The approach was always performed from the right side of the patients, due to the surgeon’s dexterity, since recurrent laryngeal nerve palsy incidence is independent of the side of the approach. A transverse skin incision was performed, through a skin fold in reference to the operated level, as marked on lateral fluoroscopy, for 2-level patients and in 3-level patients with platysma undermining. For 4-level, a longitudinal incision was preferred to expose all the extent of subaxial cervical spine. After longus colli lateral dissection, longitudinal and transversal soft-tissue blade retractors were placed. Longitudinal retractors tend to be of a smaller height than transverse ones to allow higher angle of attack of the Kerrison rongeur under the posterior vertebral body osteophytes.
After fluoroscopic confirmation of the correct level, a thorough discectomy was performed with extensive removal of anterior and posterior osteophytes and foraminal decompression, assuring proper spinal cord and nerve root decompression. Anterior osteophyte gardening is essential to allow a proper visualization of the disc space. Progressing with a “shaving” drilling technique along the disc space is essential, especially in more spondylotic discs, to keep a constant visualization of three layers: cephalad end-plate cartilage, disc and caudal end-plate cartilage—to avoid violation of the vertebral end plates. No vertebral body pins were placed for retraction with the surgeon relying instead on the suction tube for intermittent distraction of the endplates as needed. Posterior longitudinal ligament was always completely removed at disc level for inspection of putative extruded disc material posterior to the ligament, in order to properly remove osteophytes passing with the 2 mm punch under the ligament and to avoid a scaffold for later HO. At the end of the decompression, a leverage maneuver with the suction tube allowed to assess the mobility between the vertebral bodies intra-operatively and, accordingly, to insert or not a cervical disc prosthesis. Extensive cleansing with saline is performed to remove bone dust from use of drill. Special care was taken as to ensure proper placement of the implant, both in the sagittal plane as well as in the coronal plane, taking the uncus as reference and an antero-posterior fluoroscopy (Figure 1). Bone wax was systematically interposed between the prosthesis end plate and the anterior vertebral body edge left after anterior osteophytes removal.
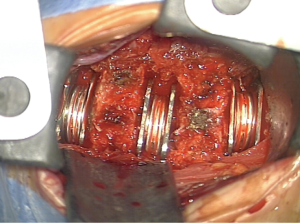
In multilevel cases, surgical decompression always started in the most compressive level to release the spinal cord compression and then progressing superiorly. The insertion of cervical disc prosthesis always started in the inferior levels progressing upwards with the aim to reconstruct disc height according to healthy individuals, since caudal discs tend to be higher than the more cephalad ones.
Study design
We performed a retrospective study including patients operated on 2-, 3- and 4-level disc disease with CDA. We used descriptive statistics to characterize patient demographics. Radiological outcome evaluations focused on pre- and post-operative neutral and dynamic (flexion and extension) cervical spine X-rays. Data was collected for the following radiological parameters: C2-7 and index segmental angles, SVA, global and segmental ROM measured using the SECTRA® (Sectra AB, Linköping, Sweden) validated imaging software. Clinical data on neck and arm VAS (nVAS, aVAS), Odom criteria, satisfaction with the clinical outcome, re-operation, complications and ASD rates were also obtained. The outcomes of 2-level CDA were compared against 3- and 4-level CDA. For patients with a follow-up longer than 2 years, an additional subgroup analysis to access the presence of HO was performed, according to the Mehren grade. The statistical analysis was performed with SPSS v23® (IBM Corp, Armonk, New York, USA), using descriptive statistics—t-test for independent variables, and paired t-test. All P values <0.05 were considered statistically significant.
Results
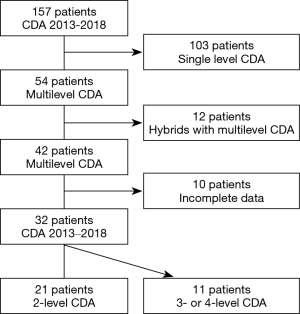
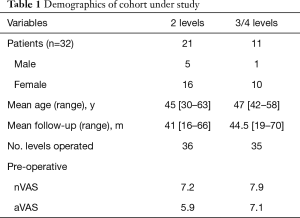
As described in Figure 2, out of 157 patients operated in our Department with CDA between 2013 and 2018, thirty-two consecutive patients were studied. Twenty-one patients underwent 2-level procedures, 9 were operated on 3 levels, and 2 patients treated at 4 levels. In total, 77 implants were placed. The demographics are as described in Table 1.
Full table
Radiological outcomes
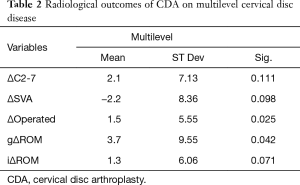
After multilevel CDA, global lordosis increased with a ΔC2-7 of 2.1±7.13º (P=0.111), as shown on Table 2. At the index level, there was a significant increment in segmental lordosis (Δindex 1.5±5.55º, P=0.025). There was a trend towards reduction in the SVA (Δ−2.2±8.36 mm, P=0.098). Global ROM improved significantly (3.7±9.55º, P=0.042), and segmental ROM showed a strong trend towards increase (1.3±6.06º, P=0.071) after multilevel CDA.
Full table
Patients with a follow-up longer than 2 years (71/77 discs), showed HO in 9.9% (7/71 discs) of cases with either grade I (8.5%) or grade II (1.4%) HO, according to the Mehren grade (22). No patient presented with severe HO, defined as grades III and IV. There was no loss of mobility in the seven discs, which developed HO, with a slight increase in ROM compared to pre-operative status (Δ3.4±8.2 mm, P=0.31).
Clinical outcomes
All patients improved in terms of nVAS and aVAS, from a mean pre-operative 7.5±1.1 to 2.5±1.5 (P<0.05), and from a mean 6.3±1.9 to 2.2±1.7 (P<0.05), respectively. According to Odom’s criteria, 90.6% (29/32) of patients achieved a favorable outcome, defined as excellent or good. Three were ranked as unfavorable with a fair outcome, and two of these stated they were not satisfied with their outcomes.
Five patients presented with post-operative transient dysphagia, 3 cases of which in 2-level surgeries and 2 in 3-level procedures. Two patients presented with hoarseness, one in a 2-level procedure and the other one a 3-level CDA. All 7 fully improved within the first post-operative month. No patients were re-operated at the index or adjacent levels.
Sub analysis: 2-level vs. 3- or 4-level CDA
Both groups presented with similar effects on sagittal balance with an increase in global lordosis (1.2±6.4º P=0.39 for 2-level, and 3.9±8.5º P=0.181 with 3 or 4 levels), and decrease in SVA (−1.3±8.08º, P=0.47 and −3.4±6.3º P=0.107, respectively) as described in Table 3.
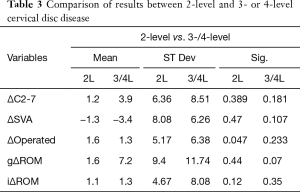
Full table
ROM also increased in both subgroups: gROM increased 1.6±9.4º (P=0.44) for 2-level patients and 7.2±11.7º (P=0.07) with 3 or 4 levels. In terms of iROM, there was a gain in mobility for both subgroups, with 2-level CDA gaining 1.1±4.7º (P=0.12), while the remaining patients gained 1.3±8.1º (P=0.35) (Table 3). When performing a direct comparison between variables in each group, there was no statistically significant change (ΔC2-7 t=−2.6, P=0.081; ΔSVA t=−2.8, P=0.065; ΔgROM t=−1.04, P=0.375; ΔiROM t=−0.208, P=0.837), showing a similar trend of variation.
Clinically, 2-level CDA improved for both nVAS and aVAS (nVAS: 7.2±1.2 to post-operative 2.7±1.5; aVAS: 5.9±1.9 to post-operative 2.1±1.6: P<0.05), with similar results for the 3- and 4-level CDA group in both scores (nVAS: 7.9±0.5 to post-operative 1.5±1.5; aVAS: 7.1±1.6 to post-operative 2.2±1.8: P<0.05). When performing a breakdown of HO results among the 2 subgroups, we find grade I HO in 8.3% (3/39) and grade II in 2.7% (1/39) of 2-level patients, compared to 8.6% (3/35) of grade I HO in 3- and 4-level patients.
Discussion
Evidence in literature to support CDA is growing with putative more favorable results than ACDF (30-33). A significant number of studies reported good clinical outcomes, both in the analysis of CDA cohorts or in comparison with ACDF. Vaccaro et al. (1) found CDA with Secure-C superior to ACDF in terms of overall success with a composite score (improvement of at least 25% in NDI, no implant failures, no complications and no pseudarthrosis for ACDF implants specifically), with 79.2% in the CDA group, against 63.6% for the ACDF group. Though not statistically superior in NDA, the CDA group showed a trend towards superiority (NDI improvement of >15 points in 88.8% vs. 84.1%) when compared with ACDF. Pointillart et al. (34) pointed out good clinical outcomes in their prospective cohort of 20 CDA patients followed for 15 years after treatment with the Bryan artificial disc with 80% of excellent outcomes according to Odom’s criteria, 2.6 for nVAS, and NDI of 14.9. Hu et al. (35) also reported an improvement in VAS (6.1±2.5 to 1.9±0.8 after 5 years), much like Zeng et al. (36), who reported improvements in both nVAS (6.0±2.2 to 20±1.4) and aVAS (6.2±2.5 to 1.9±1.4) at 6 years of follow-up, both with Prestige-LP. Two level CDA also has been reported to provide for good clinical outcomes. Gao et al. (37) stated in their 5-year study of Prestige-LP patients an improvement in VAS, from 5.3±2.3 to 1.9±1.0, along with significant improvements in NDI and SF-36. In their systematic review of literature on multilevel CDA, Joaquim et al. (38) presented the outcomes of 14 studies. In 4 out of 5, CDA either showed significant clinical superiority over ACDF or a trend in that direction. With a 90.6% favorable outcome by Odom criteria, a decrease in both nVAS (7.5±1.1 to 2.5±1.5) and aVAS (6.3±1.9 to 2.2±1.7) at end of follow-up, our results are in accordance with those described in literature. Also remarkable, is the high rate of patient satisfaction (Figure 3) with only two patients unhappy with their outcomes.
The most significant advantage attributed do CDA is motion and sagittal balance preservation. Guérin et al. (39) reported gains in both C2-7 lordosis and ROM (12.8º to 16.0º of lordosis, 8.3º to 11.0º of ROM) after single level CDA with Mobi-C, while Ahn et al. (40), in their study with ProDisc-C C5-6, concluded that global and segmental alignment of the subaxial spine became significantly more lordotic in the latter stages of follow-up, defined as over three months. These results are in-line with our analysis at two years of follow-up, almost reaching statistical significance in the increase in C2-7 lordosis and a significant increase in lordosis at the operated levels. Furthermore, a decrease in SVA (Δ−2.2±8.36, P=0.098) was found, though not significant. This is relevant for the clinical outcome given the positive clinical effect that an SVA bellow 4 cm has in terms of lower regional disability, better general health and pain scores (41,42). As for ROM, our study showed a trend towards increase, both in C2-7 the index level (ROM and ΔROM of 3.7±9.6º and 1.3±6.1º, respectively), compared to pre-operative. These results match the literature data with several studies describing either maintenance or gains in ROM after multilevel CDA (36,37,43-45). Retaining multilevel disc mobility is of paramount importance to reach favorable clinical outcomes and may exacerbate differences in clinical outcome between CDA and ACDF, especially in multilevel disc disease.
One of the key theoretical benefits of arthroplasty is the prevention of ASD when compared to fusion. Matsunaga et al. (9) analyzed the stress that fusion imposes on the adjacent levels, describing that after 2 or 3 level ACDF shear strain increased by 20% on adjacent natural discs. Eighty-five percent of the discs with increase strain went on to develop disc herniation over the follow-up period (mean 6.5 years). Park et al. (46) also found increases in disc pressure, especially on the annulus at the adjacent levels after 2-level ACDF, when compared to CDA or hybrid constructs. On distal levels, facets also suffered an increased load after 2-level ADF. Earlier on, Hilibrand et al. (11) reported 25.6% of ASD at ten years after ACDF with an incidence of 2.9 percent per year. Buttermann et al. (7) described 21% of surgery for ASD after 10 years of follow-up for ACDF, while Ishihara et al. (47) reports 19% of ASD in those patients followed for more than 2 years. Lawrence et al. (48) described a mean rate of symptomatic ASD after arthrodesis of 1.6–4.2% per year, and mean reoperation rate of 0.8% per year. In their study comparing CDA against ACDF, Gao et al. (32) reported 8.3% of ASD for CDA, whereas ACDF lead to 22.2%. Vaccaro et al. (1) reports 4.2% of surgically treated ASD with Secure-C at 84 months, compared to 16% after ACDF, though with higher rates of symptomatic ASD for both groups (17% and 37.5%, respectively). In one of the few studies directed towards the same third generation implants that we used, Jadik et al. (49) reports 2/55 patients (3.6%) were operated for symptomatic ASD. No patient in our cohort of 32 patients had symptomatic ASD leading to re-operation. Although the present study is not designed nor powered to study the reasons behind these low rates of ASD, these results are worth of notice. Shin et al. (50) reported increasing rates of ASD as more levels were fused (15.38% for 1-level ACDF, 28.57% for 2-level, and 39.47% for 3-level,), a trend which did not occur in our cohort of patients submitted to multilevel CDA.
The criticism of CDA focuses especially in HO whose incidence increases in parallel with the duration of the follow-up. Due to its effects on ROM, HO has the potential to mitigate much of the benefits of the implant. Since Mehren et al. (22) described their results for HO (33.8% grade 0 after 1 year, 19.5% of grades 3 and 4), several studies described variable rates, in particular for the highest grades. The NORCAT trial (23) described 62% of grades 3 and 4 HO, while Vaccaro et al. (1) reported only 7.7% grade 4 HO after 84 months of follow-up for the use of Secure-C for single level disc disease. The clinical impact of HO, as considered by Barbagallo et al. (51), is, however, questionable. The author stated that despite 42% rates of HO, there was a variable preservation of ROM at end of follow-up. In our cohort, we found a low-level HO with 9.9% (7/71 discs) of cervical discs followed for longer than 2 years (maximum of 70 months), showing either grade 1 or grade 2. Despite the HO that ensued, these functional units retained mobility with a slight increase in ROM at end of follow-up (Δ3.4±8.2 mm, P=0.31). The reasons for a reduced rate of HO were discussed by Tu et al. (52), who stated in 2012 that technical factors, such as postoperative shell kyphosis and inadequate endplate coverage, may increase the rate of HO (10.3% vs. 3.7% grade 4 HO, comparing suboptimal with optimal carpentry). Other factors, such as exhaustive irrigation to remove bone dust may help to reduce HO rates. Mehren et al. (53) published in 2019 the effect of implant design, namely the effect of cortical violation by the prosthesis anchoring system, concluding that it does raise rates of HO. However, there are no studies to the best of our knowledge that specifically evaluate the putative benefits of third generation cervical disc prosthesis. We believe that extensive removal of posterior longitudinal ligament abolishes the scaffold for bone growth favoring formation of HO. Extensive disc removal and insertion of larger implants to achieve better endplate coverage may also provide greater stability with a positive effect on smaller HO rates.
Our subgroup analysis between 2-level CDA and 3- or 4-level CDA demonstrated similar results, both in clinical outcome and sagittal balance, as well as global and segmental ROM outcomes (Table 3). Several studies reached similar conclusions. Goffin et al. (54), in their study of single versus 2-level CDA with Bryan cervical disc, found ROM for single-level CDA to be 7.9±5.3º, similar to 2-level CDA (7.4±5.1º). Zhao et al. (55) in their meta-analysis of eight studies found similar outcomes in clinical factors (NDI, nVAS and aVAS), HO and re-operation rates after 1 and 2 years for single and 2-level CDA. Joaquim et al. (38) in another meta-analysis which included some cohorts with 3- and 4-level patients, also reported similar equivalence, mostly in clinical, but also radiological outcomes, namely on ROM. The lack of correlation between groups in terms of effect on sagittal balance and ROM shows that 3- and 4-level CDA can improve kinematics, in a way similar to 2-level procedures.
The impact of the number of levels submitted to CDA on HO is still open to discussion. At 2 years of follow-up, Huppert et al. (45) reported similar outcomes for 1- and 2-level CDA, along with lower rates of HO in the multilevel group. Wu et al. (56), however, found similar clinical outcomes but more HO in the multilevel group after a mean follow-up of 38.3±8.7 months, raising the possibility that with enough post-operative surveillance, HO may lead to worse outcomes for multilevel patients. Though the number of 3 and 4 level patients in our study is limited to secure definitive conclusions, there is no major difference in HO rate in both subgroups. Anedoctically, 3- and 4-level patients actually had less HO versus 2-level CDA. These results, coupled with the concomitant increase in ROM in the two groups, may lead to the conclusion that an increase in number of operated levels using a restrained implant does not cause loss of benefit. In our cohort, the 3- and 4-level patients were relatively young with a mean age of 47 years. Given a long life expectancy, motion preservation and low rates of HO may contribute to a better long-term clinical outcomes compared to fusion in multilevel disc disease patients: a randomized controlled trial including only 3- and 4-level CDA patients needs to be done in the future to test this hypothesis.
Some limitations about our study should be mentioned. Although the cohorts of 3- and 4-level CDA patients are not abundantly reported in the literature, we recognize the relatively small number of patients. The present cohort includes a ratio of almost 2:1 between 2 treated levels and 3–4 levels. It is rather difficult to identify patients with pure disc disease in 3 and 4 cervical discs—cervical spondylosis does not progress evenly at all levels and it is more likely for the clinician to face single or double levels, that would benefit from CDA or a mixture of levels amenable to CDA and fusion.
On the other hand, the strong points of our study are the fact that all patients were operated by a single senior surgeon, using only one type of implant and also the long follow-up for 3- and 4-level patients.
Conclusions
CDA with third generation implant is safe and effective in 2-level disc disease, maintaining ROM and a favorable post-operative sagittal alignment profile with relatively low rates of ASD and HO. Additionally, in selected patients with more than 2 degenerated discs, CDA retains its protective effect on cervical biokinematics with excellent clinical outcomes and sagittal balance.
Acknowledgments
We would like to thank Ms. Sonia Macedo for kindly editing and reviewing this manuscript for English language.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee (nº 57/2020), and informed consent was obtained at the time of original data collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vaccaro A, Beutler W, Peppelman W, et al. Long-Term Clinical Experience with Selectively Constrained SECURE-C Cervical Artificial Disc for 1-Level Cervical Disc Disease: Results from Seven-Year Follow-Up of a Prospective, Randomized, Controlled Investigational Device Exemption Clinical Trial. Int J Spine Surg 2018;12:377-87. [Crossref] [PubMed]
- Gornet MF, Burkus JK, Shaffrey ME, et al. Cervical Disc Arthroplasty with Prestige LP Disc Versus Anterior Cervical Discectomy and Fusion: Seven-Year Outcomes. Int J Spine Surg 2016;10:24. [Crossref] [PubMed]
- Burkus JK, Traynelis VC, Haid RW Jr, et al. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine 2014;21:516-28. [Crossref] [PubMed]
- Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 2007;6:198-209. [Crossref] [PubMed]
- Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of BRYAN Cervical Disc Arthroplasty With Anterior Cervical Decompression and Fusion Clinical and Radiographic Results of a Randomized, Controlled, Clinical Trial. Spine (Phila Pa 1976) 2009;34:101-7. [Crossref] [PubMed]
- Zigler JE, Delamarter R, Murrey D, et al. ProDisc C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: five-year results of a Food and Drug Administration study. Spine (Phila Pa 1976) 2013;38:203-9. [Crossref] [PubMed]
- Buttermann GR. Anterior Cervical Discectomy and Fusion Outcomes over 10 Years: A Prospective Study. Spine (Phila Pa 1976) 2018;43:207-14. [Crossref] [PubMed]
- Grasso G, Landi A. Long-term clinical and radiological outcomes following anterior cervical di scectomy and fusion by zero-profile anchored cage. J Craniovertebr Junction Spine 2018;9:87-92. [Crossref] [PubMed]
- Matsunaga S, Kabayama S, Yamamoto T, et al. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine (Phila Pa 1976) 1999;24:670-5. [Crossref] [PubMed]
- Elsawaf A, Mastronardi L, Roperto R, et al. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev 2009;32:215-24; discussion 224. [Crossref] [PubMed]
- Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg AM 1999;81:519-28. [Crossref] [PubMed]
- Epstein NE. A Review of Complication Rates for Anterior Cervical Diskectomy and Fusion (ACDF). Surg Neurol Int 2019;10:100. [Crossref] [PubMed]
- De la Garza-Ramos R, Xu R, Ramhmdani S, et al. Long-term clinical outcomes following 3- and 4-level anterior cervical discectomy and fusion. J Neurosurg Spine 2016;24:885-91. [Crossref] [PubMed]
- Yang Y, Ma L, Liu H, et al. Comparison of the incidence of patient-reported post-operative dysphagia between ACDF with a traditional anterior plate and artificial cervical disc replacement. Clin Neurol Neurosurg 2016;148:72-8. [Crossref] [PubMed]
- Joaquim AF, Makhni MC, Riew KD. Evidence-based use of arthroplasty in cervical degenerative disc disease. Int Orthop 2019;43:767-75. [Crossref] [PubMed]
- Hu Y, Lv G, Ren S, et al. Mid- to Long Term Outcomes of Cervical Disc Arthroplasty versus Anterior Cervical Discectomy and Fusion for Treatment of Symptomatic Cervical Disc Disease: A Systematic Review and Meta-Analysis of Eight Prospective Randomized Controlled Trials. PLoS One 2016;11:e0149312. [Crossref] [PubMed]
- Zheng B, Hao D, Guo H, et al. ACDF vs TDR for patients with cervical spondylosis - an 8 year follow up study. BMC Surg 2017;17:113. [Crossref] [PubMed]
- Phillips FM, Geisler FH, Gilder KM, et al. Long-term Outcomes of the US FDA IDE Prospective, Randomized Controlled Clinical Trial Comparing PCM Cervical Disc Arthroplasty With Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2015;40:674-83. [Crossref] [PubMed]
- Xie L, Liu M, Ding F, et al. Cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) in symptomatic cervical degenerative disc diseases (CDDDs): an updated meta-analysis of prospective randomized controlled trials (RCTs). Springerplus 2016;5:1188. [Crossref] [PubMed]
- McAnany SJ, Overley S, Baird EO, et al. The 5-year cost effectiveness of anterior cervical discectomy and fusion and cervical discreplacement: a Markov analysis. Spine (Phila Pa 1976) 2014;39:1924-33. [Crossref] [PubMed]
- Kim JS, Dowdell J, Cheung ZB, et al. The Seven-Year Cost Effectiveness of Anterior Cervical Discectomy and Fusion Versus Cervical Disc Arthroplasty: A Markov Analysis. Spine (Phila Pa 1976) 2018;43:1543-51. [Crossref] [PubMed]
- Mehren C, Suchomel P, Grochulla F, et al. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976) 2006;31:2802-6. [Crossref] [PubMed]
- Sundseth J, Jacobsen EA, Kolstad F, et al. Heterotopic ossification and clinical outcome in nonconstrained cervical arthroplasty 2 years after surgery: the Norwegian Cervical Arthroplasty Trial (NORCAT). Eur Spine J 2016;25:2271-8. [Crossref] [PubMed]
- Kieser DC, Cawley DT, Fujishiro T, et al. Risk factors for anterior bone loss in cervical disc arthroplasty. J Neurosurg Spine 2018;29:123-9. [Crossref] [PubMed]
- Nunley PD, Coric D, Frank KA, et al. Cervical disc arthroplasty: current evidence and real-world application. Neurosurgery 2018;83:1087-106. [Crossref] [PubMed]
- Hey HWD, Hong CC, Long AS, et al. Is hybrid surgery of the cervical spine a good balance between fusion and arthroplasty? Pilot results from a single surgeon series. Eur Spine J 2013;22:116-22. [Crossref] [PubMed]
- Laratta JL, Shillingford JN, Saifi C, et al. Cervical Disc Arthroplasty: a comprehensive review of single-level, multilevel, and hybrid procedures. Global Spine J 2018;8:78-83. [Crossref] [PubMed]
- Segal DN, Grabel ZJ, Wilson JM, et al. Total Disk Replacement Adjacent to a Multilevel Fusion in the Cervical Spine: A Biomechanical Motion Analysis. World Neurosurg 2019;122:e881-e889. [Crossref] [PubMed]
- Jadik S, Christine Miller FC, Pietila AT. Cervical Arthroplasty with the M6-C Artificial Disc in Degenerative Disc Disease of the Cervical Spine. Arch Med 2015;7:13.
- Zhu RS, Kan SL, Cao ZG, et al. Secondary Surgery after Cervical Disc Arthroplasty versus Fusion for Cervical Degenerative Disc Disease: A Meta-analysis with Trial Sequential Analysis. Orthop Surg 2018;10:181-91. [Crossref] [PubMed]
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion: a systematic review with meta-analysis of data from a total of 3160 patients across 14 randomized controlled trials with both short- and medium- to long-term outcomes. Bone Joint J 2018;100-B:991-1001. [Crossref] [PubMed]
- Gao X, Yang Y, Liu H, et al. A Comparison of Cervical Disc Arthroplasty and Anterior Cervical Discectomy and Fusion in Patients with Two-Level Cervical Degenerative Disc Disease: 5-Year Follow-Up Results. World Neurosurg 2019;122:e1083-e1089. [Crossref] [PubMed]
- Kim BJ, Kim SH, Lee SH, et al. Segmental Motion of the Cervical Spine After Total Disc Replacement Using ActivC Versus Discectomy and Fusion Using Stand-alone Cage. World Neurosurg 2019;126:e1228-e1234. [Crossref] [PubMed]
- Pointillart V, Castelain JE, Coudert P, et al. Outcomes of the Bryan cervical disc replacement: fifteen year follow-up. Int Orthop 2018;42:851-7. [Crossref] [PubMed]
- Hu X, Jiang M, Liu H, et al. Five-Year Trends in Center of Rotation After Single Level Cervical Arthroplasty with the Prestige-LP Disc. World Neurosurg 2019;132:e941-e948. [Crossref] [PubMed]
- Zeng J, Liu H, Rong X, et al. Clinical and radiographic outcomes of cervical disc arthroplasty with Prestige-LP Disc: a minimum 6-year follow-up study. BMC Musculoskelet Disord 2018;19:285. [Crossref] [PubMed]
- Gao X, Yang Y, Liu H, et al. Cervical disc arthroplasty with Prestige-LP for the treatment of contiguous 2-level cervical degenerative disc disease: 5-year follow-up results. Medicine (Baltimore) 2018;97:e9671. [Crossref] [PubMed]
- Joaquim AF, Riew KD. Multilevel cervical arthroplasty: current evidence. A systematic review. Neurosurg Focus 2017;42:E4. [Crossref] [PubMed]
- Guérin P, Obeid I, Gille O, et al. Sagittal Alignment After Single Cervical Disc Arthroplasty. J Spinal Disord Tech 2012;25:10-6. [Crossref] [PubMed]
- Ahn PG, Kim KN, Moon SW, et al. Changes in cervical range of motion and sagittal alignment in early and late phases after total disc replacement: radiographic follow-up exceeding 2 years. J Neurosurg Spine 2009;11:688-95. [Crossref] [PubMed]
- Smith JS, Lafage V, Ryan DJ, et al. Association of myelopathy scores with cervical sagittal balance and normalized spinal cordvolume: analysis of 56 preoperative cases from the AOSpine North America Myelopathy study. Spine (Phila Pa 1976) 2013;38:S161-70. [Crossref] [PubMed]
- Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976) 2013;38:S149-60. [Crossref] [PubMed]
- Phillips FM, Tzermiadianos MN, Voronov LI, et al. Effect of two-level total disc replacement on cervical spine kinematics. Spine (Phila Pa 1976) 2009;34:E794-9. [Crossref] [PubMed]
- Goffin J, van Loon J, Van Calenbergh F, et al. A clinical analysis of 4- and 6-year follow-up results after cervical disc replacement surgery using the Bryan Cervical Disc Prosthesis. J Neurosurg Spine 2010;12:261-9. [Crossref] [PubMed]
- Huppert J, Beaurain J, Steib JP, et al. Comparison between single- and multi-level patients: clinical and radiological outcomes 2 years after cervical disc replacement. Eur Spine J 2011;20:1417-26. [Crossref] [PubMed]
- Park J, Shin JJ, Lim J. Biomechanical analysis of disc pressure and facet contact force after simulated two-level cervical surgeries (fusion and arthroplasty) and hybrid surgery. World Neurosurg 2014;82:1388-93. [Crossref] [PubMed]
- Ishihara H, Kanamori M, Kawaguchi Y, et al. Adjacent segment disease after anterior cervical interbody fusion. Spine J 2004;4:624-8. [Crossref] [PubMed]
- Lawrence BD, Hilibrand AS, Brodt ED, et al. Predicting the risk of adjacent segment pathology in the cervical spine: a systematic review. Spine (Phila Pa 1976) 2012;37:S52-64. [Crossref] [PubMed]
- Jadik S, Miller FC, Pietila AT. Cervical Arthroplasty with the M6-C Artificial Disc in Degenerative Disc Disease of the Cervical Spine. Arch Med 2015;7:13-4.
- Shin JJ. Comparison of Adjacent Segment Degeneration, Cervical Alignment, and Clinical Outcomes After One- and Multilevel Anterior Cervical Discectomy and Fusion. Neurospine 2019;16:589-600. [Crossref] [PubMed]
- Barbagallo GM, Corbino LA, Olindo G, et al. Heterotopic ossification in cervical disc arthroplasty: Is it clinically relevant? Evid Based Spine Care J 2010;1:15-20. [Crossref] [PubMed]
- Tu TH, Wu JC, Huang WC, et al. The effects of carpentry on heterotopic ossification and mobility in cervical arthroplasty: determination by computed tomography with a minimum 2-year follow-up: Clinical article. J Neurosurg Spine 2012;16:601-9. [Crossref] [PubMed]
- Mehren C, Wuertz-Kozak K, Sauer D, et al. Implant Design and the Anchoring Mechanism influence the Incidence of Heterotopic Ossification in cervical Total Disc Replacement at 2-year follow-up. Spine (Phila Pa 1976) 2019;44:1471-80. [Crossref] [PubMed]
- Goffin J, Van Calenbergh F, van Loon J, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine (Phila Pa 1976) 2003;28:2673-8. [Crossref] [PubMed]
- Zhao H, Cheng L, Hou Y, et al. Multi-level cervical disc arthroplasty (CDA) versus single-level CDA for the treatment of cervical disc diseases: a meta-analysis. Eur Spine J 2015;24:101-12. [Crossref] [PubMed]
- Wu JC, Huang WC, Tsai TY, et al. Multilevel arthroplasty for cervical spondylosis: more heterotopic ossification at 3 years of follow-up. Spine (Phila Pa 1976) 2012;37:E1251-9. [Crossref] [PubMed]