
Treatment of symptomatic thoracic disc herniations with lateral interbody fusion
Introduction
Several challenges exist in the successful surgical treatment of symptomatic thoracic herniated discs (1,2). Historically, surgical approaches have been associated with limited efficacy [decompressive laminectomy (3)], unacceptably high morbidity (4), or both. Posterior approaches limit adequate exposure of anterior pathology without spinal cord retraction or nerve root sacrifice, while anterior approaches typically require a broad exposure to access relatively small pathology. The exposure afforded by a thoracotomy approach has been considered the “gold standard” visualization for thoracic disc pathology but at the cost of elevated rates of intercostal neuralgia (5,6), diaphragmatic disfiguration, pulmonary complications (4-7), excessive blood loss (7-9), and infection (6,8). Endoscopic approaches to the anterior thoracic column have eliminated much of the morbidity associated with thoracotomy though, unfortunately, this has been replaced with a new profile of surgical challenges including a steep and long learning curve (10), extensive and expensive instrumentation and staffing, the potential for emergent conversion to open exposures, visualization of three dimensional anatomy in two dimensions, and difficulty in placing anterior instrumentation (10-12).
Modern minimally disruptive approaches for thoracolumbar pathology have been developed under direct visualization using specialized access systems, standard surgical techniques, and small incision exposures with limited soft tissue dissection (2,13-16). In recent years, the direct lateral approach has become a popular technique for treating lumbar pathologies, although its application in the thoracic spine is not widely reported. The aim of this study was to present a representative case series of a mini-open lateral approach for anterior thoracic access in the treatment of symptomatic thoracic herniated discs.
Methods
Study design
A prospective registry was analyzed retrospectively for three patients who underwent a minimally invasive lateral approach for thoracic discectomy and interbody fusion (XLIF®, NuVasive®, Inc. San Diego, CA, USA) by a single surgeon (GMM). Data collected included patient (demographic and indication), treatment (operative and complications), and outcome (clinical, radiographic, return to work, and functional improvements). Radiographic evidence of fusion was assessed using high definition computed tomography (CT) (Somatom Definition Flash, Siemens AG, Erlangen, Germany) at 6- and 12-month follow-up. Fusion was defined as the presence of bridging interbody trabecular bone according to Williams et al. (17). All patients were followed for 24 months postoperatively.
Surgical technique
The surgical technique for both lumbar and thoracic lateral interbody fusion (LIF) has been previously reported (2,15,16,18), and can be used in the treatment of a variety of degenerative conditions of the thoracolumbar spine from approximately T4 to L5, blocked superiorly by the axilla and inferiorly by the iliac crest. Briefly, the patient is treated in the lateral decubitus position on a radiolucent operative table with a true lateral and anteroposterior orientation orthogonal to the floor, confirmed on fluoroscopy. The table break should be placed more superior than for a lumbar approach to allow for some separation of the ribs on the approach side during lateral flexion under table break. The side of the approach is dictated by both the presenting pathology and relevant vascular anatomy. The target vertebral level was marked at the beginning of the operation by placing a Kirschner wire in the spinous process immediately inferior to the pathological thoracic disc to reduce the risk of wrong level surgery. Motor evoked potentials were monitored intraoperatively (NV M5®, NuVasive, Inc., CA, USA) and thus, total intravenous anesthesia was used to avoid attenuation of motor pathway signals.
The approach begins with skin incision approximately 4-5 cm in length, parallel with and between the ribs, 90 degrees lateral to the disc space. Blunt dissection, aided by periosteal elevators and doyens, through the intercostal muscles at the superior margin of the inferior rib is performed to encourage preservation of the neuromuscular bundle.
For solitary thoracic pathology, a transpleural approach was employed by incising the parietal pleura, introducing the initial dilator, coursing down the rib to the rib head over the target disc space, verified by lateral fluoroscopy. For pathologies at the thoracolumbar junction, a retropleural transdiaphragmatic approach was performed and care was taken to preserve the visceral pleura to avoid pneumothorax and/or haemothorax. Following exposure into the space, a series of three sequential dilators and a self retaining split blade retractor (MaXcess IV® retractor, NuVasive, Inc., CA, USA) are introduced and secured to the operating table by a rigid articulating arm. Once the desired exposure is achieved, decompression, endplate preparation, and intervertebral polyetheretherketone (PEEK) implant placement (CoRoent XL-T®, NuVasive, Inc., CA, USA) are performed using standard operative techniques. Preoperative examination of axial magnetic resonance imaging (MRI) can aid in the identification of any vascular anatomy (aorta) with respect to the degree of contralateral annular release possible.
Interbody spacers were filled with a combination of bone morphogenetic protein (rhBMP-2, Infuse®, Medtronic, Inc., Memphis, TN, USA) and Mastergraft β-TCP granules (Medtronic, Inc., Minneapolis, USA). The cages were sealed with Surgicel® (Ethicon, Inc. Somerville, NJ, USA) or fat graft, supplemented with tissue glue to avoid extravasation of rhBMP-2 into the thoracic cavity. Chest tubes can be placed at the surgeon’s discretion.
Results
Patient demographic and treatment information is summarized in Table 1. Clinical and radiographic outcomes are summarized in Table 2.
Table 1
| Case no. | Age | Sex | BMI | Indication | Level | Side | Bone graft | Chest tube | EBL (mL) | LOS (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | Male | 33.9 | TDH | T6-7 | Right | rhBMP-2 | No | < 50 | 5 |
| 2 | 62 | Female | 27.4 | TDH | T9-10 | Left | rhBMP-2 | No | < 50 | 5 |
| 3 | 74 | Male | 24.6 | DDD | T12-L1 | Left | rhBMP-2 | Yes | < 50 | 5 |
BMI, body mass index; DDD, degenerative disc disease; EBL, estimated blood loss; LOS, length of stay; rhBMP-2, recombinant human Bone Morphogenetic Protein-2; TDH, thoracic disc herniation.
Table 2
| Case no. | Complications | Follow-up | Fusion | Return to work (full-time) | Preoperative | 24-month follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | ODI | PCS | MCS | VAS | ODI | PCS | MCS | ||||||
| 1 | None | 24 months | 6 months | 3 months | 10 | 84 | 11 | 54 | 1 | 0 | 50 | 55 | |
| 2 | Neuropathic thoracic pain | 24 months | 6 months | 6 months | 9 | 56 | 33 | 44 | 1.5 | 24 | 48 | 62 | |
| 3 | None | 24 months | 12 months | 5 weeks | 8 | 38 | 33 | 46 | 2 | 20 | 40 | 52 | |
MCS, mental component score; ODI, Oswestry Disability Index; PCS, physical component score; VAS, visual analogue scale.
Case 1
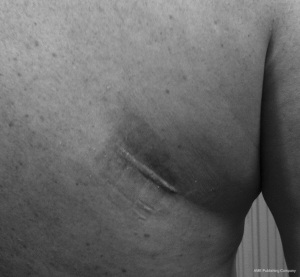
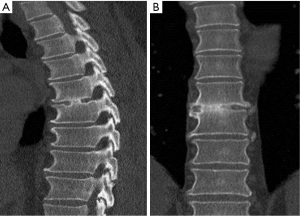
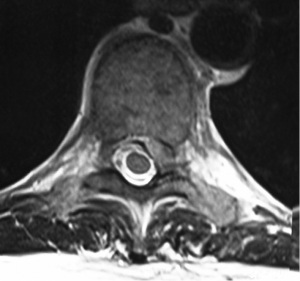
A 49-year-old male presented with a 4-day history of progressive myelopathy with bladder and bowel dysfunction, unsteadiness and leg numbness. He had no medical comorbidities and was a non-smoker. Examination demonstrated spastic gait, positive Romberg’s test, lower limb hyperreflexia with midthoracic sensory level and saddle anesthesia. Thoracolumbar MRI revealed an acute central T6-7 soft central disc prolapse compressing the spinal cord causing intrinsic high cord signal (Figure 1). MRI brain excluded mass lesion and demyelination. Two days of 16 mg dexamethasone treatment was provided without symptom response or improvement. Hence, the patient underwent a right transpleural T6-7 LIF. After complete channel discectomy, a ball tipped hook was used to remove the extruded central disc fragment through a rent in the ruptured posterior longitudinal ligament (PLL). The disc space was distracted with sequential interbody spacer trials until the appropriate height was achieved, followed by cage placement (6×16×35 mm3, 10 degree, CoRoent XL-T). Estimated blood loss (EBL) was less than 50 mL. A postoperative chest tube was not required for drainage. Postoperative chest radiography excluded pneumothorax and CT confirmed cord decompression and satisfactory prosthesis placement at T6-7. The patient exhibited rapid resolution of myelopathic symptoms and was discharged 5 days postoperatively. At 6 weeks postoperative the skin incision was well healed with excellent cosmesis (Figure 2) and he was no longer taking analgesic medication. The patient returned to light work duties at 8 weeks and then to full duties 3 months postoperatively. Solid fusion was confirmed on CT at 6-month follow-up (Figure 3). The patient reported a vast improvement in pain, from a visual analogue scale (VAS) of 10/10 preoperatively, to 1/10 at 24-month follow-up. There were also improvements in Oswestry Disability Index (ODI) (84 to 0), and SF-36 physical component score (PCS) (11 to 50) and mental component score (MCS) (54 to 55).
Case 2
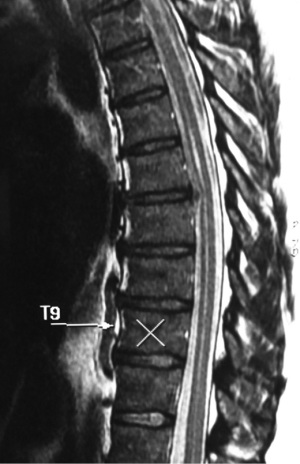
A 62-year-old female presented with a 3-week history of severe left lower thoracic radicular pain. The patient had no neurological deficit on examination. She had no medical comorbidities and was a non-smoker. Initial chest CT and ultrasound were unremarkable. MRI thoracolumbar scan showed an acute, moderate sized, left sided T9-10 foraminal soft disc prolapse extending superiorly into the left T9-10 foramen with significant compression of the exiting left T9 nerve root (Figure 4). The patient was unresponsive to opiate analgesia, pregabalin (Lyrica®, Pfizer, New York, NY, USA) and CT guided left T9 transforaminal epidural nerve root injection. The patient underwent a left sided transpleural T9-10 LIF. Following formal discectomy, a ball tipped blunt hook was inserted superiorly into the T9-10 foramen for removal of extruded disc fragments. A standalone interbody cage (6×16×40 mm3, zero degree, CoRoent XL-T) was placed. EBL was less than 50 mL. A chest tube was not required. Postoperative chest radiography excluded pneumothorax. CT showed satisfactory prosthesis placement at T9-10 with no residual neural compression (Figure 5). The patient reported reduction in preoperative thoracic pain (VAS 9/10 to 5/10), further improved to 2-3/10 with a CT guided T9-10 epidural injection. She was discharged to home 5 days after surgery. At 3 months postoperative, the patient continued to exhibit mild persistent neuropathic thoracic pain requiring paracetamol and pregabalin 75 mg daily, but had returned to part-time work duties. At 6-month follow-up she had solid interbody fusion, confirmed on CT, and had resumed full-time work. At 24 months follow-up the patent reported further improvement in pain (VAS 1-2/10); her ODI (56 to 24), PCS (33 to 48) and MCS (44 to 62) were also improved.
Case 3
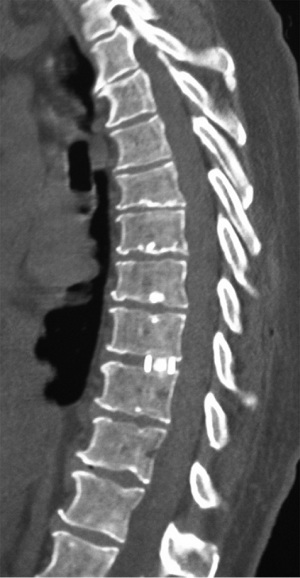
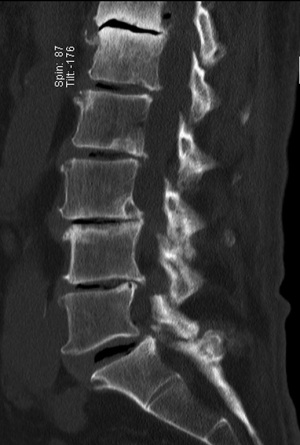
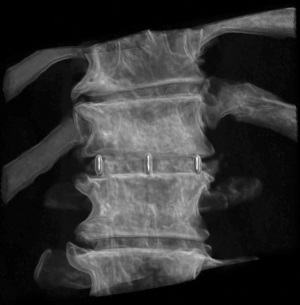
A 74-year-old male presented with a 12-month history of progressive low back pain (VAS 8/10) with an inability to stand for more than 10 minutes or participate in recreational activities. No neurologic deficit was revealed on initial examination, comorbidities included hypertension and prior history of tobacco use. Preoperative CT thoracolumbar scan demonstrated multilevel degenerative disc disease (DDD) most marked at T12-L1 with a degenerative grade I spondylolisthesis at L3-4 (Figure 6). Isotope Tc99m bone scan coregistered with CT showed radiotracer uptake mainly at T12-L1 disc level and lesser extent at L3-4 disc and L5-S1 facet joints. The patient was unresponsive to opiate analgesia, CT guided facet joint or transforaminal epidural nerve root injections. The patient underwent a left retropleural transdiaphragmatic T12-L1 LIF with an interbody cage (8×18×45 mm3, zero degree, CoRoent XL-T). EBL was less than 50 mL. A 24 gauge intercostal drain was placed intraoperatively and removed 24 hours postoperatively. Postoperative CT demonstrated satisfactory prosthesis placement at T12-L1. The patient was discharged to home on day 5. He ceased all analgesia and returned to normal work duties 5 weeks postoperatively. A solid fusion was confirmed on CT at 12 months (Figure 7). The patient reported improvements in low back pain (VAS 8/10 to 2/10), ODI (38 to 20), PCS (33 to 40) and MCS (46 to 52) at 24 month follow-up.
Discussion
Symptomatic thoracic disc herniation requiring surgical intervention is rare (19), representing only a small portion of spinal surgeries for all disc herniations (20,21). Conventional open surgical approaches have high complication rates and approach related morbidity, which extends recovery and return to normal activities (6,9,22,23).
Historically, the surgical treatment of thoracic disc herniations was approached posteriorly with decompressive laminectomy (24). In 1969, Perot and Munro reviewed the literature on the treatment of thoracic disc herniation with laminectomy and found that of the 91 patients reported at the time, only 56% experienced either partial or complete symptom resolution, with 18% being rendered paraplegic and 7% dead (24). From these early failures, anterior approaches were adopted to better address thoracic disc pathology, namely central and calcific discs. While thoracotomy has been suggested to be the “gold standard” visualization for thoracic pathology, the associated morbidity negates much of the benefits. Following thoracotomy, overall complication rates have been reported as high as 150% (5) with blood loss measured in liters (7,22), length of hospital stay measured in weeks (6,22,25,26), and post thoracotomy pain persisting in as many as 30% of patients 5 years postoperatively (27).
Modern minimally disruptive procedures that use specialized retractors for direct visualization and standard surgical techniques with minimal soft tissue dissection have emerged as viable options for thoracic discectomy (2,13-16). In the current study, three patients were successfully treated with a mini-open lateral approach for treatment of degenerative thoracic pathology (Tables 1,2). No intraoperative complications were observed and in each case, EBL was less than 50 mL. All patients were discharged 5 days postoperatively. Two patients experienced full symptom resolution and returned to full-time work by 3 months postoperative. One patient reported mild persistent pain postoperatively, but returned to full-time work at 6 months. Two patients were fused at 6-month follow-up and the remaining patient was fused at 12 months. At 24 month follow-up patients reported mean improvements of 83.3% in VAS, 75.3% in ODI, and 79.2% and 17.4% in SF-36 PCS and MCS, respectively.
Three contemporary studies have reported similar outcomes using this approach for thoracic pathology. Deviren et al. (15) reported outcomes in 12 patients who were treated with LIF for thoracic disc herniations with an average follow-up of 28 months. All patients had chest tubes inserted for 24 hours postoperatively. Average hospital stay was 5 days. Two complications were observed: one pleural effusion and one case of intercostal neuralgia. Patient pain (VAS) improved from 9 to 3 (67%) preoperatively to last follow-up. All 8 patients with preoperative myelopathy improved postoperatively.
In a 22 patient study by Karikari et al. (28) three patients were treated for thoracic disc herniations. Average blood loss and hospital stay were 67 mL and 3.7 days, respectively. Peri or postoperative complications were not encountered through an average of 17 months follow-up. All patients had evidence of radiographic fusion. Improvement in VAS averaged 46% (8.3 to 4.5) from preoperative to last follow-up.
In a multicenter study by Uribe et al. (2), 60 consecutive patients underwent LIF for thoracic disc herniation with an average follow-up of 11 months. Median operative time was 182 minutes with 290 mL blood loss and 5 days hospital stay. Minor complications occurred in 7% of patients. Four patients had major complications including pneumonia, extrapleural free air, new lower extremity weakness, and wound infection in posterior hardware. From preoperative to last follow-up, VAS improved 60% (7.8 to 3.1). Symptomatology substantially improved: myelopathy improved in 83%, radiculopathy improved in 87%, and back pain improved in 91% of cases. At last follow-up, 80% of patients experienced “excellent” or “good” overall outcomes, with 15% exhibiting “fair” or “unchanged” outcomes, and only 5% categorized as “poor”.
Direct lateral surgery has proven to be an effective procedure when treating lumbar pathologies, given the minimally disruptive approach and preservation of stabilizing, anatomical structures. This study is a representative case series of commonly seen thoracic pathologies, central cord compression, nerve root compression and symptomatic disc degeneration, with which this technique can be adopted.
Conclusions
Treatment of thoracic degenerative disease with a minimally invasive lateral technique for discectomy and interbody fusion has only recently been reported. Our results are consistent with the literature in showing both efficacy in symptom resolution and fusion, but with substantially decreased procedural morbidity compared to conventional open approaches. Additionally, the procedure can be performed without an access surgeon, rib resection or lung deflation. Although only a limited number of cases are reported in this study, the positive outcomes presented are encouraging for the future treatment of thoracic disc pathologies with the LIF approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: GM. Malham has received travel support from Medtronic, NuVasive and Stryker.
References
- Williams MP, Cherryman GR, Husband JE. Significance of thoracic disc herniation demonstrated by MR imaging. J Comput Assist Tomogr 1989;13:211-4. [PubMed]
- Uribe JS, Smith WD, Pimenta L, et al. Minimally invasive lateral approach for symptomatic thoracic disc herniation: initial multicenter clinical experience. J Neurosurg Spine 2012;16:264-79. [PubMed]
- Perez-Cruet MJ, Kim BS, Sandhu F, et al. Thoracic microendoscopic discectomy. J Neurosurg Spine 2004;1:58-63. [PubMed]
- Khoo LT, Smith ZA, Asgarzadie F, et al. Minimally invasive extracavitary approach for thoracic discectomy and interbody fusion: 1-year clinical and radiographic outcomes in 13 patients compared with a cohort of traditional anterior transthoracic approaches. J Neurosurg Spine 2011;14:250-60. [PubMed]
- Johnson JP, Filler AG, Mc Bride DQ. Endoscopic thoracic discectomy. Neurosurg Focus 2000;9:e11. [PubMed]
- Rosenthal D, Dickman CA. Thoracoscopic microsurgical excision of herniated thoracic discs. J Neurosurg 1998;89:224-35. [PubMed]
- Hott JS, Feiz-Erfan I, Kenny K, et al. Surgical management of giant herniated thoracic discs: analysis of 20 cases. J Neurosurg Spine 2005;3:191-7. [PubMed]
- Machino M, Yukawa Y, Ito K, et al. A new thoracic reconstruction technique "transforaminal thoracic interbody fusion": a preliminary report of clinical outcomes. Spine (Phila Pa 1976) 2010;35:E1000-5. [PubMed]
- Regan JJ, Ben-Yishay A, Mack MJ. Video-assisted thoracoscopic excision of herniated thoracic disc: description of technique and preliminary experience in the first 29 cases. J Spinal Disord 1998;11:183-91. [PubMed]
- Khoo LT, Beisse R, Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurgery 2002;51:S104-17. [PubMed]
- McAfee PC, Regan JR, Zdeblick T, et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine (Phila Pa 1976) 1995;20:1624-32. [PubMed]
- McAfee PC, Regan JR, Fedder IL, et al. Anterior thoracic corpectomy for spinal cord decompression performed endoscopically. Surg Laparosc Endosc 1995;5:339-48. [PubMed]
- Bartels RH, Peul WC. Mini-thoracotomy or thoracoscopic treatment for medially located thoracic herniated disc? Spine (Phila Pa 1976) 2007;32:E581-4. [PubMed]
- Chi JH, Dhall SS, Kanter AS, et al. The Mini-Open transpedicular thoracic discectomy: surgical technique and assessment. Neurosurg Focus 2008;25:E5. [PubMed]
- Deviren V, Kuelling FA, Poulter G, et al. Minimal invasive anterolateral transthoracic transpleural approach: a novel technique for thoracic disc herniation. A review of the literature, description of a new surgical technique and experience with first 12 consecutive patients. J Spinal Disord Tech 2011;24:E40-8. [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [PubMed]
- Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol 2005;26:2057-66. [PubMed]
- Deviren V, Pekmezci M, Tay B. Thoracic disc herniation: extreme lateral approach. In: Goodrich JA, Volcan IJ. eds. Extreme Lateral Interbody Fusion (XLIF). St. Louis: Quality Medical Pub, 2008;239-59.
- Russell T. Thoracic intervertebral disc protrusion: experience of 67 cases and review of the literature. Br J Neurosurg 1989;3:153-60. [PubMed]
- Awwad EE, Martin DS, Smith KR Jr, et al. Asymptomatic versus symptomatic herniated thoracic discs: their frequency and characteristics as detected by computed tomography after myelography. Neurosurgery 1991;28:180-6. [PubMed]
- Wood KB, Blair JM, Aepple DM, et al. The natural history of asymptomatic thoracic disc herniations. Spine (Phila Pa 1976) 1997;22:525-9; discussion 529-30. [PubMed]
- Bransford R, Zhang F, Bellabarba C, et al. Early experience treating thoracic disc herniations using a modified transfacet pedicle-sparing decompression and fusion. J Neurosurg Spine 2010;12:221-31. [PubMed]
- Epstein JA. The syndrome of herniation of the lower thoracic intervertebral discs with nerve root and spinal cord compression. A presentation of four cases with a review of literature, methods of diagnosis and treatment. J Neurosurg 1954;11:525-38. [PubMed]
- Perot PL Jr, Munro DD. Transthoracic removal of midline thoracic disc protrusions causing spinal cord compression. J Neurosurg 1969;31:452-8. [PubMed]
- Albrand OW, Corkill G. Thoracic disc herniation. Treatment and prognosis. Spine (Phila Pa 1976) 1979;4:41-6. [PubMed]
- Ayhan S, Nelson C, Gok B, et al. Transthoracic surgical treatment for centrally located thoracic disc herniations presenting with myelopathy: a 5-year institutional experience. J Spinal Disord Tech 2010;23:79-88. [PubMed]
- Karmakar MK, Ho AM. Postthoracotomy pain syndrome. Thorac Surg Clin 2004;14:345-52. [PubMed]
- Karikari IO, Nimjee SM, Hardin CA, et al. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: initial clinical experience and early outcomes. J Spinal Disord Tech 2011;24:368-75. [PubMed]


