
The role of perioperative ketamine in postoperative pain control following spinal surgery
Introduction
The current opioid epidemic
In recent years opioid misuse and abuse have become an epidemic in the United States. 80% of the world’s opioid supply is now consumed by the US alone (1). In 2015, it was reported that 2 million people were addicted to prescription opioids, and a staggering 12 million reported opioid misuse (2). Deaths caused by prescription opioid overdose annually have nearly quadrupled from 1999−2010 (3) with a 124% increase in unintentional prescription opioid deaths seen from 2004 to 2008 (4). A significant relationship was seen with overdose deaths and maximum prescribed daily dose of opioid medication (4), and those with a history of substance abuse were at almost 14 times greater risk (5). In addition to the cost of life, several studies have also looked at the growing economic burden—one study estimated that opioid misuse costs the US $50 billion annually, a large majority in lost productiveness followed by criminal justice costs (6).
Narcotics in postoperative pain control
A major gateway through which people are introduced to opioids is through postoperative care as patients. As a result of these trends, the United States surgeon general issued a recent call to action. This call to action aims to focus on prescription monitoring programs, responsible prescribing, recognition of addiction in patients, and the de-stigmatization of those battling opioid addiction. In 2014, the FDA also reclassified hydrocodone to a schedule II substance from a schedule III substance to help curb overprescribing (7).
Although this trend affects patients undergoing all different types of procedures, orthopaedic surgery is one of the largest impacted. A recent study found that among postoperative patients undergoing various surgeries (appendectomy, cholecystectomy, cesarean delivery, sinus surgery, cataract surgery, prostate surgery, mastectomy, total hip arthroplasty and total knee arthroplasty), patient undergoing total knee arthroplasty were 5.1 times more likely to develop chronic opioid use, the highest in the study (8). 7.7% of all opioid prescriptions in the US in 2009 were written by orthopaedic surgeons (9). Opioid overprescribing disproportionately affects orthopedic surgery, and spine surgery in particular, given the nature of the field. Generally, longstanding pain and difficulty with activities of daily living lead to orthopedic spine referrals. Patients that eventually undergo surgery are often those that have failed conservative management with medications, physical therapy and/or injections. The procedure itself, especially in large deformity corrections, also necessitates substantial pain control regimens in the perioperative period. Though medications exist to help patients in the treatment of opioid addiction such as buprenorphine and naltrexone (9), as orthopedic surgeons we can aid in fighting this epidemic and preventing addiction by setting patient expectations, identifying at-risk patients, and finding alternatives to opioids for postoperative pain control (10).
Ketamine in postoperative pain control
Finding alternatives to opioids in postoperative pain control is critical in reducing opioid dependence following discharge; one such alternative that has been gaining popularity recently has been ketamine. Ketamine is an NMDA receptor antagonist, a glutamate receptor in the nociceptive pathway (11). With the advent of S(+)-ketamine form, ketamine in sub-anesthetic doses for pain control has been increasing in use (11). This form of ketamine has been shown to have a 4 times stronger affinity for NMDA, with increased clinical analgesic effects (12,13). Pain stimuli peripherally lead to NMDA receptor activation—this may lead to windup phenomenon, in which repetitive stimulation can cause prolonged increase in neuron excitability, increasing subjective pain (14). This may then lead to central sensitization and pain memory; interrupting this pathway is the principle by which ketamine plays a role in pain control (14).
A common concern with the use of ketamine has been its side effects seen in drug abuse including agitation, hallucinations, and confusion (15). In a large meta-analysis of ketamine adjuncts to opioid for pain control which included 37 randomized double-blind clinical trials of 2,385 patients overall, various central nervous system side effects in using ketamine as a pain control adjunct have been reported including (dizziness, diplopia, dysphoria, dreams, hallucinations, strange sensation, lightheadedness, sleep difficulties, confusion) (16). However, no significant increase was seen compared to those patients who did not receive ketamine and only opioids for pain control; if anything decreased postoperative nausea and vomiting were associated with the addition of ketamine (16).
Ketamine has been explored in a wide variety of surgical settings. A recent large meta-analysis found that ketamine was more effective in reducing opioid burden in surgeries with more postoperative pain (higher VAS scores) (17). For example, upper abdominal and thoracic procedures showed the greatest decrease in opioid consumption while dental, or head and neck surgery showed a smaller response (17). Within orthopedic surgery, more involved procedures such as spine surgery showed a greater benefit with ketamine those smaller procedures such as arthroscopy (17). As such, we hope to review the most up-to-date prospective clinical trials published on ketamine use in spine surgery to evaluate its efficacy and potential in reducing opioid dependence postoperatively.
Methodology
A PubMed search was performed querying “ketamine”, “spine, and “surgery”. A total of 70 articles resulted, of which 11 prospective randomized control trials were identified. In order to standardize the prospective studies included in this review, several inclusion criteria were established. All studies included patients undergoing an elective spinal procedure (cervical, thoracic, or lumbar were all included). Both pediatric and adult spinal procedures were included. All studies were required to have clearly defined intraoperative analgesia dosing. Specific dosing and timing of ketamine both intraoperatively and postoperatively had to be described. Lastly, patient-controlled analgesia (PCA) had to be utilized in the postoperative period in order to quantify the primary endpoint of opioid consumption. Based on these criteria, 10 of the 11 prospective randomized control trials identified were included in this review.
Of the studies included, ketamine was administered either as a bolus at incision, a continuous infusion throughout the procedure, or both (Table 1). Medication administered by PCA varied between studies and included morphine, hydromorphone, fentanyl, and methadone (Table 1). Study enrollment ranged from 22 to 202 subjects, and average age ranged from 14 to 61. All studies included were published between 2004 and 2017.
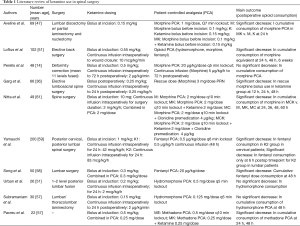
Full table
Ketamine in spine surgery
When looking specifically at spine surgery, the combination of morphine and ketamine has been explored the most in recent randomized control trials. One of the earliest studies to randomize spine surgery patients undergoing elective surgical lumbar discectomy with partial laminectomy and nucleotomy for ketamine administration was performed in 2005 (18). Sixty-nine patients were randomized into three different groups: patients receiving only morphine (control), only ketamine, or a combination of ketamine with morphine as an IV bolus just after induction of anesthesia. Aveline et al. found that the combined ketamine/morphine (KM) group consumed significantly less IV morphine in the PACU than the morphine only (M) group (P=0.009), however no significant difference was seen between the KM group and the ketamine only (K) group (18). Postoperative pain scores (VAS) were significantly lower in the combined KM group versus either the K or M groups. They also found significantly lower incidence of postoperative nausea and vomiting in the KM group versus the M group. Here we see a single dose of ketamine significantly reduce opioid consumption without a significant increase in side effects; this allows one to consider an expanded role for ketamine including as a continuous infusion throughout surgery or even continued into the postoperative period in conjunction with traditional morphine PCAs, which was explored in subsequent randomized control trials.
In 2010, Loftus et al. similarly looked at 101 patients undergoing spine surgery who were randomized to receive either IV ketamine on induction with an infusion until wound closure, or to a control group who only received normal saline during the case. A PCA (either hydromorphone, fentanyl, or morphine) was ordered for all patients postoperatively. Those who received intraoperative ketamine consumed significantly less morphine equivalents postoperatively than the control group at 24 hours (30% reduction, P=0.032) and 48 hours (48% reduction, P=0.029) (19). Corresponding decreases in PACU pain intensity were also seen in the PACU and at 6 weeks follow-up (P=0.033, P=0.026 respectively) (19). Of note, this study found a 71% reduction in opiate consumption at 6 weeks follow-up (P=0.041) (19). No significant differences were seen in side effects including nausea, constipation, or hallucinations (19).
This study went further to compare the effect on patients after stratifying for preoperative opioid use. Interestingly, after stratifying for preoperative opioid use they found a significant reduction in morphine use at 24 and 48 hours postoperatively in the ketamine group, only in those with a significant history of preoperative opioid use (19). This study explores several key variables: side effects, preoperative opioid use status, and outpatient follow up at 6 weeks. Again, no significant difference is seen in side effects with the use of ketamine. This study also highlights the importance of preoperative screening and a detailed understanding of preoperative opioid use in determining outcomes. Lastly, we see potential long-term benefits of ketamine as illustrated by the decrease in opioid consumption over 6 weeks which has significant implications broadly across all fields.
A study by Perello et al. looked at the pediatric population undergoing spine surgery for AIS, with patients ages 10 to 18. They looked at morphine consumption, pain at rest and movement, side effects, onset of oral intake, onset of ambulation, and length of stay for scoliosis surgery. Patients were randomized into a control group and a ketamine group: one group received an IV bolus of ketamine at induction as well as continuous IV infusion until 72 hours postoperatively; the control group received the equivalent in normal saline. Although no significant differences were seen in this population with total cumulative morphine consumption, secondary outcomes such as pain at rest or during movement, nausea, vomiting, itching, or dysphoria, hallucinations, nightmares, diplopia, or respiratory depression also had no significant difference than the control group, which speaks to concerns regarding the safety of ketamine in analgesia (especially in younger patients) (20).
Other studies included comparisons of morphine with other combinations of medications. One recent study in 2016 by Garg et al. combined morphine with either ketamine and midazolam or a dexmedetomidine drip. The cumulative morphine requirement, as well as mean pain-free period, was significantly lower with the ketamine and dexmedetomidine groups relative to a control group, with no significant increase in relevant side effects (21). Another study by Nitta et al. combined morphine PCA with either a control infusion, clonidine premedication, ketamine intraoperatively and postoperatively, or ketamine with the addition of clonidine premedication, and found that the morphine/clonidine/ketamine group had a significantly lower cumulative morphine requirement than the morphine only group starting 12 hours postoperatively. Pain scores at rest, at 24 hours postoperatively and at 48 hours postoperatively for the morphine and morphine/ketamine group were lower than those receiving clonidine, however pain scores with movement had no significant differences (22).
Ketamine in combination with other PCA medications other than morphine have also been looked at. A large study in 2008 looked at 202 patients undergoing either cervical or lumbar surgery. Patients were on a fentanyl drip with PCA demand dosing with the addition of either ketamine at 42 or 83 mcg/kg/h. Patients were also able to request NSAIDs every 8 hours as well. This study added another level of complexity by stratifying patients based on anatomic location of surgery. Cervical patients had significantly lower pain scores at the higher ketamine dose versus the control group or the lower ketamine dose group at 24 hours postoperatively. Fentanyl consumption was also significantly lower in the higher ketamine dose group. NSAID consumption in the higher ketamine dose group was also lower for cervical patients. For lumbar patients, the higher ketamine dose group showed significantly lower fentanyl consumption only at 6 hours. This study looked at several variables not previously addressed including variable ketamine dosing, anatomic surgical location, as well as supplemental NSAID use (23). A similar study looked at patients on a fentanyl-based PCA versus those given ketamine IV bolus after induction in addition to ketamine in the fentanyl PCA. This study focused on side effects, namely postoperative nausea/vomiting. They found that the incidence was similar in a high-risk group (female, non-smoking patients). They also found significantly lower fentanyl consumption over 48 hours in the ketamine group (24).
When combined with a hydromorphone PCA, a smaller study of 26 patients undergoing 1 or 2 level lumbar fusions (narcotic-tolerant) found that patients given ketamine at induction and for the following 24 hours had significantly less pain in the first postoperative hour and at post-operative day 1 (POD1), both at rest and while working with physical therapy (25). Though the ketamine group did require less hydromorphone overall, the differences were not statistically significant (25). Another study looked at hydromorphone PCAs in combination with ketamine infusion both intraoperatively and for the first 24 hours postoperatively; all patients in this study additionally received epidural bupivacaine in the surgery however. This study on the other hand found no difference in pain scores at rest or movement, or differences in hydromorphone consumption between the control and ketamine groups (26).
The final combination studied was the use of ketamine in conjunction with a methadone PCA. Ketamine was administered in a bolus after intubation and as a continuous infusion throughout the surgery. Pacreu et al. found that the methadone with ketamine (MK) group required more remifentanil at induction versus methadone only, however the MK group attempted less doses with PCA postoperatively. The MK group received 70% less methadone via PCA at 24 hours and significantly less at 48 hours postoperatively as well. The ketamine group also had attempted significantly less doses (27).
Of the 10 prospective studies included in this study, 7 trials found a significant decrease in opioid consumption postoperatively at varying timepoints when ketamine was incorporated in some form into the analgesia regimen (18,19,21-24,27). On the other hand, 3 of the prospective studies reviewed found no significant change in opioid consumption (20,25,26), including the only study performed exclusively in a pediatric population (20). In all these studies, no significant side effects were seen with the addition of sub-anesthetic doses of ketamine either intraoperatively or postoperatively. 2 of the studies stratified for those patients who were opioid-tolerant preoperatively and found lower opioid consumption (19) and decreased pain scores postoperatively (25).
Conclusions
The opioid epidemic continues to grow in the United States and will require a multi-faceted approach to address, from identifying vulnerable patients to implementing effective addiction recovery options. As surgeons, one major contribution that can be made is at the front end, to reduce the number of people exposed to opioids in the first place - decreasing the need for pain medication in the postoperative period and finding alternatives to opioids will be critical towards reaching this goal. Ketamine has shown great promise in this respect, specifically following spine surgery. Randomized control trials exploring the efficacy of ketamine in postoperative pain control following spine surgery have only been sporadically performed over the last 15 years, and though the results have varied slightly most of the studies show significant reduction in postoperative opioid consumption with the adjunct of ketamine with minimal adverse side effects. The studies reviewed here illustrate the complexity and difficulty in performing a comprehensive study, addressing variables such as dosing, timing, administration, and supplemental medication. With further study of ketamine and other medication alternatives to opioids, the hope is to eliminate postoperative pain control as a gateway into the ever-growing opioid crisis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-19-306). LGL reports personal fees from Medtronic, grants and personal fees from DePuy-Synthes Spine, personal fees from K2M, non-financial support from Broadwater, non-financial support from Seattle Science Foundation, grants and non-financial support from Scoliosis Research Society, non-financial support from Stryker Spine, non-financial support from The Spinal Research Foundation, grants from EOS, grants from Setting Scoliosis Straight Foundation, personal fees from Fox Rothschild, LLC, personal fees from Quality Medical Publishing, other from Evans Family Donation, other from Fox Family Foundation, grants and non-financial support from AOSpine, outside the submitted work; Dr. Lehman reports personal fees from Medtronic, personal fees from Stryke Synthes Spine, grants from Department of Defense grant for research support, outside the submitted work. LGL serves as an unpaid editorial board member of Journal of Spine Surgery from Oct 2019 to Oct 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manchikanti L, Singh A. Therapeutic opioids: A ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008;11:S63-88. [PubMed]
- Han B, Compton WM, Blanco C, et al. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017;167:293-301. [Crossref] [PubMed]
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA 2013;309:657-9. [Crossref] [PubMed]
- Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305:1315-21. [Crossref] [PubMed]
- Cauley CE, Anderson G, Haynes AB, et al. Predictors of In-hospital Postoperative Opioid Overdose After Major Elective Operations: A Nationally Representative Cohort Study. Ann Surg. 2017;265:702-8. [Crossref] [PubMed]
- Hansen RN, Oster G, Edelsberg J, et al. Economic costs of nonmedical use of prescription opioids. Clin J Pain 2011;27:194-202. [Crossref] [PubMed]
- Jones CM, Lurie PG, Throckmorton DC. Effect of US Drug Enforcement Administration's Rescheduling of Hydrocodone Combination Analgesic Products on Opioid Analgesic Prescribing. JAMA Intern Med 2016;176:399-402. [Crossref] [PubMed]
- Sun EC, Darnall BD, Baker LC, et al. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 2016;176:1286-93. [Crossref] [PubMed]
- Volkow ND, McLellan TA, Cotto JH, et al. Characteristics of opioid prescriptions in 2009. JAMA 2011;305:1299-301. [Crossref] [PubMed]
- Morris BJ, Mir HR. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg 2015;23:267-71. [Crossref] [PubMed]
- Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology 2005;102:211-20. [Crossref] [PubMed]
- Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: Evidence for a role of N -methyl-d-aspartate receptors. J Pharmacol Exp Ther 1992;260:1209-13. [PubMed]
- Arendt-Nielsen L, Nielsen J, Petersen-Felix S, et al. Effect of racemic mixture and the (S+)-isomer of ketamine on temporal and spatial summation of pain. Br J Anaesth 1996;77:625-31. [Crossref] [PubMed]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991;44:293-9. [Crossref] [PubMed]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction 2012;107:27-38. [Crossref] [PubMed]
- Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg 2004;99:482-95. [Crossref] [PubMed]
- Laskowski K, Stirling A, McKay WP, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 2011;58:911-23. [Crossref] [PubMed]
- Aveline C, Hetet HL, Vautier P, et al. Peroperative ketamine and morphine for postoperative pain control after lumbar disk surgery. Eur J Pain 2006;10:653-8. [Crossref] [PubMed]
- Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010;113:639-46. [PubMed]
- Perelló M, Artés D, Pascuets C, et al. Prolonged Perioperative Low-Dose Ketamine Does Not Improve Short and Long-term Outcomes After Pediatric Idiopathic Scoliosis Surgery. Spine (Phila Pa 1976) 2017;42:E304-12. [Crossref] [PubMed]
- Garg N, Panda NB, Gandhi KA, et al. Comparison of Small Dose Ketamine and Dexmedetomidine Infusion for Postoperative Analgesia in Spine Surgery--A Prospective Randomized Double-blind Placebo Controlled Study. J Neurosurg Anesthesiol 2016;28:27-31. [Crossref] [PubMed]
- Nitta R, Goyagi T, Nishikawa T. Combination of oral clonidine and intravenous low-dose ketaminereduces the consumption of postoperative patient-controlled analgesia morphine after spine surgery 2013;51:14-7.
- Yamauchi M, Asano M, Watanabe M, et al. Continuous low-dose ketamine improves the analgesic effects of fentanyl patient-controlled analgesia after cervical spine surgery. Anesth Analg 2008;107:1041-4. [Crossref] [PubMed]
- Song JW, Shim JK, Song Y, et al. Effect of ketamine as an adjunct to intravenous patient-controlled analgesia, in patients at high risk of postoperative nausea and vomiting undergoing lumbar spinal surgery. Br J Anaesth 2013;111:630-5. [Crossref] [PubMed]
- Urban MK, Ya Deau JT, Wukovits B, et al. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS J 2008;4:62-5. [Crossref] [PubMed]
- Subramaniam K, Akhouri V, Glazer PA, et al. Intra- and postoperative very low dose intravenous ketamine infusion does not increase pain relief after major spine surgery in patients with preoperative narcotic analgesic intake. Pain Med 2011;12:1276-83. [Crossref] [PubMed]
- Pacreu S, Fernández Candil J, Moltó L, et al. The perioperative combination of methadone and ketamine reduces post-operative opioid usage compared with methadone alone. Acta Anaesthesiol Scand 2012;56:1250-6. [Crossref] [PubMed]


