Trends in lumbar spinal fusion—a literature review
Introduction
Since the earliest description of spinal fusion performed by Dr. Russell A. Hibbs in 1911 and later by Dr. Fred H. Albee for tuberculosis, it has become one of the most commonly performed orthopedic procedures. The indications for spinal fusion have broadened over time since the surgery was initially developed for the treatment of instability due to tuberculosis or deformities. Surgical techniques have evolved in the past decades and spinal fusion is now used to treat a variety of indications such as traumatic injuries, deformities, primary and secondary tumors, infections and degenerative conditions of the spine (1-3). The most common diagnoses in 2008 for spinal fusion procedures were lumbar degenerative disc disease and cervical disc replacement in the United States (13.8% and 12.2%) (1,4).
Several studies and systematic reviews have been published to provide guidelines about the optimal surgical treatment option for various indications. Various studies have showed that spinal fusion procedures have a positive effect on patient outcomes. The Spine Patient Outcomes Research Trial (SPORT) has been one of the most influential studies to investigate the treatment effect of operative and non-operative therapies in the treatment of spinal stenosis and degenerative spondylolisthesis. Published data continue to demonstrate the benefit of operative spine fusion interventions for these conditions at 2, 4 and 8 years postoperatively (5-7). Additionally, for tumor patients with spinal cord compression caused by spinal metastases, decompression combined with instrumentation showed better results compared to decompression alone (8-12).
Over the past several decades, there has been an upward trend in the total number of spinal fusion procedures worldwide. Kim et al. reported a difference in spine surgery utilization among Japan, Korea and the United States with the highest incidence of spine surgery in the United States (13). In Canada, an upward trend of lumbar fusion procedures has been reported with an increase from 6.2 to 14.2 procedures per 100,000 population between 1993 and 2012 in Ontario (14). In Australia, the number of spinal fusion procedures increased by 169% in the public and private sectors (2% and 167%) between 1997 and 2006, which was a higher increase than hip or knee arthroplasty procedures (15). In the United Kingdom, recent data showed a similar upward trend (16,17). Between 2005 and 2015, Provaggi et al. reported an increase of 63% in spinal fusion procedures in the United Kingdom (16,17). Similarly, Grotle et al. reported a significant increase in simple and complex lumbar spine surgery, mostly for fusion procedures, in Norway from 1999 to 2013 (18).
Trends in lumbar fusion procedures
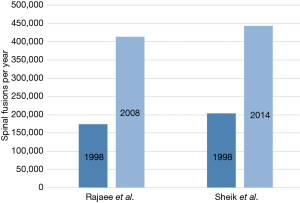
In 2004, Deyo et al. published an article on the growing use of spinal fusion procedures in the United States with a 77% increase between 1996 and 2001 (2). Rajaee et al. analyzed the spinal fusion rate between 1998 and 2008 and reported an ongoing increase in the frequency and utilization of spinal fusion in the United States with a 2.4-fold (137%) increase. Sheik et al. analyzed 7.1 million cases between 1998 and 2014 from the largest United States inpatient health-care database and showed a continuously upward trend for spinal fusion procedures (P<0.001) with an increase of 118% from 1998 to 2014 and an overall downward trend in the utilization of non-fusion spinal procedures like decompression (Figure 1) (4,19,20). In regards to differentiating the trend in the number of levels operated in lumbar fusion procedures, Al Jammal reported a higher increase in short fusion procedures than long fusion procedures between 2010 and 2014 (from 35.3% to 47.2% versus 5.7% to 7.1%) in patients with lumbar stenosis (20).
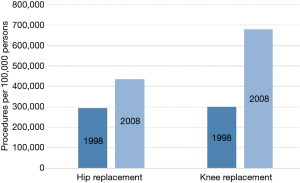
Sheik et al. reported close parallels in the upward trend of the utilization of spinal fusion as well as hip and knee procedures in the United States from 1998 to 2014, with a relative increase of 89% for spinal fusion and 81% for hip and knee procedures (4). Rajaee et al. published similar results for hip and knee arthroplasty with an increase of 49.1% and 126.8% compared to the 137% increase for spine fusion procedures between 1998 and 2008 (Figure 2) (1). The authors reported spinal fusion procedures went from the 37th most common procedure in 1998 to the 16th in 2008, directly after primary hip replacement (15th most common procedure) (1). The increase in spinal fusion procedures does not appear to be associated with higher surgery utilization rates across specialties (1,4).
Comparing cervical, thoracic and lumbar spine fusion procedures, the overall number of fusions procedures has increased in all regions of the spine. Wang et al. described a significant increase in cervical fusions of 206% for degenerative changes from 1992 to 2005 (21). Rajaee et al. reported a 114% increase in the annual number of primary cervical fusion cases and an 82% increase in primary thoracic fusion cases from 1998 to 2008 in the United States (1,4). With a 2.7-fold increase (170.9%), primary lumbar fusion had the largest increase compared to cervical and thoracic fusion procedures in this time period according to Rajaee et al. (1).
Al Jammal et al. reported a continuous increase in lumbar fusion surgeries from 41% to 54.3% in patients with lumbar stenosis in combination with and without coexisting scoliosis from 2010 and 2014 in the United States (20). Varshneya et al. reported an increase of 168.5% specifically for anterior lumbar interbody fusion (ALIF) procedures in the United States from 2007 to 2014 (22).
A multitude of factors may have contributed to increased spinal fusion utilization rates such as the improved biomechanical and pathophysiology understanding of the human spine, improved diagnostic imaging techniques, broader indications for surgery, the development of various instrumentation techniques with an increased availability of spinal fixation devices, the development of minimally invasive surgery (MIS), new surgical techniques, and innovative alternatives in bone grafting materials. Furthermore, significant technological advancements, a growing number of well-trained spine specialists, an increase in the life expectancy of the population, and the overall improved safety profile of spinal fusion procedures over time could be additional reasons for an ongoing upward trend in spine fusion surgery (1,4,13). The overall increased safety of spine fusion procedures with lower complication rates may also explain a decrease in reoperation rates (18).
Nevertheless, it is important for surgeons to be aware of potential complications and their management such as neurological injuries, dural tears, implant-related issues, pseudarthrosis, infections, and wound issues, especially in multilevel spinal fusions that have a higher risk for complication risk (23,24). Despite an increasing number of spine fusion procedures it is highly important to make individual decisions for each patient with considerations of the patient’s condition and risk for complications.
Trends in surgical approaches and implants
Advanced spinal fusion techniques like the use of pedicle screw fixation for posterior instrumentation, innovations in surgical approaches, and novel implants are factors in the increased spinal fusion rates and clinical outcomes over the last several decades (25-32). Surgical fusion is an effective treatment method to correct deformity, stabilize painful segment movement and restore lordosis and sagittal balance (31). There is a wide range of fusion methods, from anterior, lateral or posterior approaches, interbody fusion with stand-alone cages or with internal fixation that are used based on the surgical indication, surgeon preference, and patient condition.
Over the years, pedicle fixation systems for spinal stabilization have evolved to incorporate biomechanical principles of spinal stability and improvements in new technologies and materials. The systems can differ in many aspects such as in their method of attachment to the spine, specific pedicle screw design (mono- vs. poly-axial), the connection of the screw-rod system (side- vs. top-loading) as well as in the biomaterials used (33-35).
When the FDA approved intervertebral fusion cages in 1996, there was a rapid growth in fusion rates for all spinal fusion procedures (36). The variety of interbody fusion implants increased and are based on implant geometry like cage width, length, thickness and lordotic angle, material and material surface (14,37). Developments in cage designs evolved from biologic implants like bone dowels or femoral ring allografts to threaded titanium cages and polyetheretherketone (PEEK) cage devices (30). For the optimization of cage design, current studies are focusing on improving cage geometry, cage material and surface materials for better osseointegration and postoperative outcomes (35,38,39).
For posterior fusion with instrumentation, the preexisting posterolateral fusion without implants made progress over the last decades, leading to posterior lumbar interbody fusion (PLIF) as one of the most established lumbar fusion procedures. Other approaches have also been developed such as transforaminal lumbar interbody fusion (TLIF) for direct unilateral access to the intervertebral foraminal space (13,31,40). Similar to the development of posterior fusion techniques, anterior and lateral approaches have also evolved. The typical transperitoneal approach has been adapted to an anterior retroperitoneal approach.
Lumbar spinal fusion procedures are now well-known and widely adopted as surgical treatment for various spinal disorders such as congenital or degenerative deformities, degenerative disc disease, spondylolisthesis, spinal stenosis, trauma, infection and tumor (16,31). Currently there are different approaches to lumbar interbody fusion including ALIF, PLIF, lateral or extreme lateral lumbar interbody fusion (LLIF), and TLIF (Table 1).
The anterior retroperitoneal approach, mostly suitable for L4/5 and L5/S1, allows an efficient anterior discectomy and maximal implant size without injuring the posterior neural structures for effective correction of lordosis and height restoration of the affected level (31). The ALIF procedure spares the posterior and psoas muscle that improves postoperative stability, but the technique is known for visceral and vascular injuries (41,42).
PLIF is one of the traditional approaches for lumbar interbody fusion and is commonly performed by the majority of spine surgeons. The technique allows for posterior access to the spine with visualization of the nerve roots, the option for neural decompression, good interbody height restoration, and the possibility of a 360-degree fusion through a single incision (31,43). Disadvantages of the PLIF technique is that paraspinal muscle injury could result in delayed postoperative recovery, approach related injuries like retrograde ejaculation and sympathetic injury, the aggravated endplate preparation due to the posterior anatomy of the spine compared to anterior preparation, and possible inadequate correction of coronal imbalance and lordosis restoration (31,37,44).
The LLIF technique accesses the affected disc level through a lateral retroperitoneal, transpsoas approach. Due to the location of the iliac crest bone, LLIF is not suitable for treatment of the L5/S1 level. At the more caudal levels of the lumbar spine, the risk of injury to the lumbar plexus and iliac vessels increases. Advantages of this approach are less muscle injury with a potential for faster postoperative mobilization as well as the possibility for sagittal and coronal deformity correction (31,45-48).
TLIF provides direct unilateral access to the intervertebral foraminal space through the posterior spine while sparing the ligamentous structures and paraspinal muscles for better postoperative biomechanical stability. Endplate preparation can be difficult with this approach and correction of coronal imbalance and lordosis restoration is limited (31,41,49-53).
There is still no evidence on the clinical superiority of one approach over another. Indications for each approach vary based on surgeon preference, spinal fusion levels, and the patient condition (31,54,55). These fusion approaches can also be performed using mini-open or MIS techniques as a less-invasive surgical method. The evolution of MIS techniques is one possible explanation for the number of increasing spinal fusion procedures worldwide (16,31,47,56). Several studies have shown that interbody fusions have more than doubled in the past decade with a growing popularity in MIS techniques especially in PLIF and TLIF for various indications (57-60).
The lack of worldwide or even national guidelines and evidence for the treatment of different surgical indications has led to wide variability in surgical management. Studies are needed to assess clinical outcomes, reoperation and revision rates to better define the indications and efficacy of lumbar spinal fusion procedures (20,36,61-63). A worldwide comparison between increases in short and multilevel spine fusion rates would offer additional procedural evidence that can be informative about the potential impact on surgical outcomes. In addition, definitive conclusions regarding the advantages and disadvantages of a given implant and clinical evidence are lacking. Future surgical practice would benefit from continued biomechanical studies, experimentation and clinical studies. Additionally, the implementation of a spine implant registry similar to the knee and hip joint replacement registry would be useful to identify possible reasons for implant failure and improve fusion outcomes. The area of customized, patient-specific spinal implants is another interesting area and has yet to be explored in clinically relevant studies (16,64,65).
Trends in biologics
With the worldwide increasing number of spine fusion procedures performed every year, a number of new bone graft substitutes has been introduced to improve spine fusion rates as alternate methods to autograft harvesting (66-69). Bone graft substitutes such as bone marrow aspirate (BMA), mesenchymal stem cells (MSCs), allograft (i.e., cortico-cancellous allograft), and demineralized bone matrix (DBM) are often used in combination with synthetic grafts, ceramics or growth factors such as recombinant human bone morphogenetic proteins (rhBMPs) (66-68,70,71). The biologics differ in their capability as osteogenic (bone growth), osteoconductive (promotes ingrowth of blood vessels), and osteoinductive (promotes differentiation of stem cells) (72,73). Provaggi et al. showed that autograft is still the preferred bone grafting procedure in the United Kingdom (16). In contrast, there has been a small shift from autologous to other bone grafts in the United States (74).
Tissue engineering products such as bone growth factors, have the potential to lower the rate of pseudarthrosis. rhBMP-2 is currently the only FDA-approved growth factor. Additionally, recombinant human parathyroid hormone (rhPTH) and rhBMP-7 have been studied clinically for improving spinal fusion (75-78).
Autologous iliac crest bone graft (ICBG) remains the current “gold standard” as bone graft material in lumbar fusion surgery. The fusion rates with alternative bone graft substitutes like BMP-2 is still unclear and fusion rates range widely between studies. (67,75,79). Several studies investigated the complication spectrum of BMP-2, including carcinogenicity, and reported a wide range of potential complications, complication rates, and controversial conclusions (80-85). The systematic review of Mariscal et al. recently reviewed six high-quality randomized clinical trials. The review concluded that BMP-2 had more beneficial effects on posterolateral lumbar fusion rates with reduced surgical morbidity (surgical time, intraoperative blood loss, hospitalization days) with the same clinical patient-reported outcome scores (Oswestry Disability Index, 36-Item Short Form Health Survey, Back Pain Score) than ICBG in concordance with previous findings (79,86). Lao et al. reported in their trend analysis of rhBMP utilization in single-level PLIF and ALIF in the United States, a 3-fold increase from 2005 to 2011 in the rate of PLIF without rhBMP-2 compared to PLIF with rhBMP-2. There was a sharp decrease between 2009 and 2011 for ALIF with rhBMP-2 (87,88). The systematic review of Morris et al. showed comparable outcomes for most bone graft materials with the highest fusion rates for local autograft in combination with BMA in posterolateral lumbar fusion (67).
Cottrill et al. reviewed studies about experimental growth factors in animal models and identified several growth factors that may improve spine fusion rates (75). One of the most innovative research areas in spinal fusion is gene therapy. Gene therapy investigates the expression of genes that code for osteoinductive and osteogenic factors and how to target them. To date, however, gene therapy studies have been limited to preliminary animal models (89,90). Another interesting research area is the interaction of BMPs and BMP antagonists. How BMP antagonists influence BMPs’ osteogenic effects is one possible explanation for non-union after spinal fusion procedures (91-93).
With the growing number of bone graft substitutes, it is becoming increasingly difficult to compare all the available options. Consequently, deciding on the appropriate bone graft or bone graft substitute choice for the practicing spine surgeon is more challenging than in the past. Developing new technical innovations are expensive and often associated with limited use based on indication. For instance, bone graft substitutes like BMP-2 therefore are often used off-label for certain indications or surgical approaches in lumbar fusion procedures (94,95). The higher costs and restricted approval for the use of new bone grafts can prohibit their utilization despite improved clinical outcomes. An example where newer biologics may be cost prohibitive is in Germany, where the health-care system does not reimburse the costs for the usage of novel bone graft substitutes like for BMP-2 in Germany (96,97). Further studies should therefore investigate the best grafting options based on the patient and surgery while considering the overall cost-effectiveness and efficacy.
Conclusions
Spine surgery fusion rates continue to increase worldwide as a result of new developments in spine fusion procedures and surgical techniques, improved implants and interbody devices, and advancements in complication prevention strategies. Lumbar degenerative disc disease is the most common diagnosis for spine fusion surgery.
Continuous improvement of the safety profile of lumbar fusion surgery, the increasing number of minimally invasive procedures and innovative instrumentation devices in combination with new bone grafting materials will be reasons for an ongoing number of fusion procedures in the future.
How the increasing upward trend will affect the healthcare systems worldwide is one of the important future questions. Sheik et al. reported overall charges for spinal fusion procedures concurrently increased with the number of fusions from 34% in 1998 to 61% in 2014. In 1998 estimated $12 billion were charged in for spinal fusions compared to $48 billion in 2014 (4). The authors described changes in distribution of payers for spinal fusion procedures with a growing proportion of the cases paid by Medicare, from in 1998 21% of the procedures to almost 40% in 2014. Interestingly, Sheik et al. showed in contrast to the increased hospital charges a relative decrease in the reimbursement from public payers for spinal fusion procedures in the United States (4). How increasing costs of instrumentation and procedure specific costs have an impact on overall charges for spinal fusion procedures in the future and how the ongoing upward trend will be influenced is an interesting topic for future studies as well.
In the current literature, there is great variability in reporting about the ideal surgical management strategy based on indications and clinical outcomes. The results from studies about the optimal spine fusion procedure, use of instrumentation for internal fixation, instrumentation type, graft source, fusion location and postoperative treatment often conflict. Due to a lack of high-level evidence and clear guidelines, it can be difficult to compare treatment options and decide on the best surgical management. Large randomized and prospective studies are warranted to investigate fusion surgical treatment options based on patient condition and specific indications. It would also be interesting to investigate the utilization of the type of spinal fusion for specific indications and compare the upward trend between surgical procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection” published in Journal of Spine Surgery. The article was sent for external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-492). The series “Postoperative Spinal Implant Infection” was commissioned by the editorial office without any funding or sponsorship. MP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Spine Surgery from Nov 2018 to Nov 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67-76. [Crossref] [PubMed]
- Deyo RA, Nachemson A, Mirza SK. Spinal-Fusion Surgery - The Case for Restraint. N Engl J Med 2004;350:722-6. [Crossref] [PubMed]
- Camillo FX. Arthrodesis of the Spine. In: Campbell WC, Canale ST, Beaty JH. Campbell's operative orthopaedics. 11th edition. Philadelphia, PA: Mosby/Elsevier, 2008.
- Sheikh SR, Thompson NR, Benzel E, et al. Can We Justify It? Trends in the Utilization of Spinal Fusions and Associated Reimbursement. Neurosurgery 2020;86:E193-202. [PubMed]
- Ilyas H, Udo-Inyang I Jr, Savage J.. Lumbar Spinal Stenosis and Degenerative Spondylolisthesis: A Review of the SPORT Literature. Clin Spine Surg 2019;32:272-8. [Crossref] [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295-304. [Crossref] [PubMed]
- Abdu WA, Sacks OA, Tosteson ANA, et al. Long-Term Results of Surgery Compared With Nonoperative Treatment for Lumbar Degenerative Spondylolisthesis in the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 2018;43:1619-30. [Crossref] [PubMed]
- Laufer I, Sciubba DM, Madera M, et al. Surgical management of metastatic spinal tumors. Cancer Control 2012;19:122-8. [Crossref] [PubMed]
- Prasad D, Schiff D.. Malignant spinal-cord compression. Lancet Oncol 2005;6:15-24. [Crossref] [PubMed]
- Khan SN, Donthineni R. Surgical management of metastatic spine tumors. Orthop Clin North Am 2006;37:99-104. [Crossref] [PubMed]
- Eleraky M, Papanastassiou I, Vrionis FD. Management of metastatic spine disease. Curr Opin Support Palliat Care 2010;4:182-8. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery--international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir (Tokyo) 2010;50:853-8. [Crossref] [PubMed]
- Xu Y, Yen D, Whitehead M, et al. Use of instrumented lumbar spinal surgery for degenerative conditions: trends and costs over time in Ontario, Canada. Can J Surg 2019;62:393-401. [Crossref] [PubMed]
- Harris IA, Dao ATT. Trends of spinal fusion surgery in Australia: 1997 to 2006. ANZ J Surg 2009;79:783-8. [Crossref] [PubMed]
- Provaggi E, Capelli C, Leong JJH, et al. A UK-based pilot study of current surgical practice and implant preferences in lumbar fusion surgery. Medicine (Baltimore) 2018;97:e11169. [Crossref] [PubMed]
- Sivasubramaniam V, Patel HC, Ozdemir BA, et al. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: a 15-year time-series study. BMJ Open 2015;5:e009011. [Crossref] [PubMed]
- Grotle M, Småstuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 2019;9:e028743. [Crossref] [PubMed]
- Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 2013;38:916-26. [Crossref] [PubMed]
- Al Jammal OM, Delavar A, Maguire KR, et al. National Trends in the Surgical Management of Lumbar Spinal Stenosis in Adult Spinal Deformity Patients. Spine (Phila Pa 1976) 2019;44:E1369-78. [Crossref] [PubMed]
- Wang MC, Kreuter W, Wolfla CE, et al. Trends and variations in cervical spine surgery in the united states: Medicare beneficiaries, 1992 to 2005. Spine (Phila Pa 1976) 2009;34:955-61. [Crossref] [PubMed]
- Varshneya K, Medress ZA, Jensen M, et al. Trends in Anterior Lumbar Interbody Fusion in the United States: A MarketScan Study From 2007 to 2014. Clin Spine Surg 2020;33:E226-30. [PubMed]
- Al-Mohrej OA, Aldakhil SS, Al-Rabiah MA, et al. Surgical treatment of adolescent idiopathic scoliosis: Complications. Ann Med Surg (Lond) 2020;52:19-23. [Crossref] [PubMed]
- Yavin D, Casha S, Wiebe S, et al. Lumbar Fusion for Degenerative Disease: A Systematic Review and Meta-Analysis. Neurosurgery 2017;80:701-15. [Crossref] [PubMed]
- Denaro V, Di Martino A.. Cervical spine surgery: An historical perspective. Clin Orthop Relat Res 2011;469:639-48. [Crossref] [PubMed]
- Riew KD, Rhee JM. The use of titanium mesh cages in the cervical spine. Clin Orthop Relat Res 2002.47-54. [Crossref] [PubMed]
- Kandziora F, Pflugmacher R, Schäfer J, et al. Biomechanical comparison of cervical spine interbody fusion cages. Spine (Phila Pa 1976) 2001;26:1850-7. [Crossref] [PubMed]
- Wen Z, Lu T, Wang Y, et al. Anterior cervical corpectomy and fusion and anterior cervical discectomy and fusion using titanium mesh cages for treatment of degenerative cervical pathologies: A literature review. Med Sci Monit 2018;24:6398-404. [Crossref] [PubMed]
- Brown JK, Timm W, Bodeen G, et al. Asynchronously Calibrated Quantitative Bone Densitometry. J Clin Densitom 2017;20:216-25. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Evolution of Design of Interbody Cages for Anterior Lumbar Interbody Fusion. Orthop Surg 2016;8:270-7. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Bono CM, Lee CK. Critical Analysis of Trends in Fusion for Degenerative Disc Disease over the Past 20 Years: Influence of Technique on Fusion Rate and Clinical Outcome. Spine (Phila Pa 1976) 2004;29:455-63. [Crossref] [PubMed]
- Goel VK, Ebraheim NA, Biyani A, et al. Role of mechanical factors in the evaluation of pedicle screw type spinal fixation devices. Neurol India 2005;53:399-407. [Crossref] [PubMed]
- Kabins MB, Weinstein JN. The History of Vertebral Screw and Pedicle Screw Fixation. Iowa Orthop J 1991;11:127-36.
- Warburton A, Girdler SJ, Mikhail CM, et al. Biomaterials in Spinal Implants: A Review. Neurospine 2020;17:101-10. [Crossref] [PubMed]
- Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441-5. [Crossref] [PubMed]
- Fan SW, Hu ZJ, Fang XQ, et al. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg 2010;2:194-200. [Crossref] [PubMed]
- MacBarb RF, Lindsey DP, Bahney CS, et al. Fortifying the Bone-Implant Interface Part 1: An In Vitro Evaluation of 3D-Printed and TPS Porous Surfaces. Int J Spine Surg 2017;11:15. [Crossref] [PubMed]
- Rao PJ, Pelletier MH, Walsh WR, et al. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg 2014;6:81-9. [Crossref] [PubMed]
- Rickert M, Rauschmann M.. Interbody fusion procedures. Orthopade 2015;44:103. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Malham GM, Parker RM, Ellis NJ, et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine 2014;21:851-60. [Crossref] [PubMed]
- Lestini WF, Fulghum JS, Whitehurst LA. Lumbar spinal fusion: advantages of posterior lumbar interbody fusion. Surg Technol Int 1994;3:577-90. [PubMed]
- Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118-26. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989. [Crossref] [PubMed]
- Arnold PM, Anderson KK, McGuire RA Jr. The lateral transpsoas approach to the lumbar and thoracic spine: A review. Surg Neurol Int 2012;3:S198-215. [Crossref] [PubMed]
- Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg 2007;15:321-9. [Crossref] [PubMed]
- Phan K, Rao PJ, Scherman DB, et al. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci 2015;22:1714-21. [Crossref] [PubMed]
- Audat Z, Moutasem O, Yousef K, et al. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J 2012;53:183-7. [PubMed]
- Sakeb N, Ahsan K. Comparison of the early results of transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in symptomatic lumbar instability. Indian J Orthop 2013;47:255-63. [Crossref] [PubMed]
- Park JS, Kim YB, Hong HJ, et al. Comparison between Posterior and Transforaminal Approaches for Lumbar Interbody Fusion. J Korean Neurosurg Soc 2005;37:340-4.
- Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379-86. [Crossref] [PubMed]
- Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 2001;26:567-71. [Crossref] [PubMed]
- Talia AJ, Wong ML, Lau HC, et al. Comparison of the different surgical approaches for lumbar interbody fusion. J Clin Neurosci 2015;22:243-51. [Crossref] [PubMed]
- Lee N, Kim KN, Yi S, et al. Comparison of Outcomes of Anterior, Posterior, and Transforaminal Lumbar Interbody Fusion Surgery at a Single Lumbar Level with Degenerative Spinal Disease. World Neurosurg 2017;101:216-26. [Crossref] [PubMed]
- Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci 2012;19:829-35. [Crossref] [PubMed]
- Makanji H, Schoenfeld AJ, Bhalla A, et al. Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J 2018;27:1868-76. [Crossref] [PubMed]
- Thirukumaran CP, Raudenbush B, Li Y, et al. National Trends in the Surgical Management of Adult Lumbar Isthmic Spondylolisthesis: 1998 to 2011. Spine (Phila Pa 1976) 2016;41:490-501. [Crossref] [PubMed]
- Kepler CK, Vaccaro AR, Hilibrand AS, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976) 2014;39:1584-9. [Crossref] [PubMed]
- Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J 2015;15:265-71. [Crossref] [PubMed]
- Strube P, Putzier M, Siewe J, et al. To fuse or not to fuse: a survey among members of the German Spine Society (DWG) regarding lumbar degenerative spondylolisthesis and spinal stenosis. Arch Orthop Trauma Surg 2019;139:613-21. [Crossref] [PubMed]
- Taylor VM, Deyo RA, Cherkin DC, et al. Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976) 1994;19:1207-12; discussion 13. [Crossref] [PubMed]
- Weinstein JN, Lurie JD, Olson PR, et al. United States' trends and regional variations in lumbar spine surgery: 1992-2003. Spine (Phila Pa 1976) 2006;31:2707-14. [Crossref] [PubMed]
- Serra T, Capelli C, Toumpaniari R, et al. Design and fabrication of 3D-printed anatomically shaped lumbar cage for intervertebral disc (IVD) degeneration treatment. Biofabrication 2016;8:035001. [Crossref] [PubMed]
- Capelli C, Serra T, Kalaskar D, et al. Computational models for characterisation and design of patient-specific spinal implant. Spine J 2016;16:S53-4. [Crossref]
- Stark JR, Hsieh J, Waller D. Bone graft substitutes in single-or double-level anterior cervical discectomy and fusion: A systematic review. Spine (Phila Pa 1976) 2019;44:E618-28. [Crossref] [PubMed]
- Morris MT, Tarpada SP, Cho W. Bone graft materials for posterolateral fusion made simple: a systematic review. Eur Spine J 2018;27:1856-67. [Crossref] [PubMed]
- Yoo JS, Ahn J, Patel DS, et al. An evaluation of biomaterials and osteobiologics for arthrodesis achievement in spine surgery. Ann Transl Med 2019;7:S168. [Crossref] [PubMed]
- Campana V, Milano G, Pagano E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 2014;25:2445-61. [Crossref] [PubMed]
- D'Souza M, Macdonald NA, Gendreau JL, et al. Graft Materials and Biologics for Spinal Interbody Fusion. Biomedicines 2019;7:75. [Crossref] [PubMed]
- Greene AC, Hsu WK. Orthobiologics in minimally invasive lumbar fusion. J Spine Surg 2019;5:S11-8. [Crossref] [PubMed]
- Oryan A, Alidadi S, Moshiri A, et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 2014;9:18. [Crossref] [PubMed]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001;10 Suppl 2:S96-101. [Crossref] [PubMed]
- Kinaci A, Neuhaus V, Ring DC. Trends in bone graft use in the United States. Orthopedics 2014;37:e783-8. [Crossref] [PubMed]
- Cottrill E, Ahmed AK, Lessing N, et al. Investigational growth factors utilized in animal models of spinal fusion: Systematic review. World J Orthop 2019;10:176-91. [Crossref] [PubMed]
- Ebata S, Takahashi J, Hasegawa T, et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J Bone Joint Surg Am 2017;99:365-72. [Crossref] [PubMed]
- Ohtori S, Orita S, Yamauchi K, et al. More than 6 Months of Teriparatide Treatment Was More Effective for Bone Union than Shorter Treatment Following Lumbar Posterolateral Fusion Surgery. Asian Spine J 2015;9:573-80. [Crossref] [PubMed]
- Ye F, Zeng Z, Wang J, et al. Comparison of the use of rhBMP-7 versus iliac crest autograft in single-level lumbar fusion: a meta-analysis of randomized controlled trials. J Bone Miner Metab 2018;36:119-27. [Crossref] [PubMed]
- Mariscal G, Nuñez JH, Barrios C, et al. A meta-analysis of bone morphogenetic protein-2 versus iliac crest bone graft for the posterolateral fusion of the lumbar spine. J Bone Miner Metab 2020;38:54-62. [Crossref] [PubMed]
- Hustedt JW, Blizzard DJ. The controversy surrounding bone morphogenetic proteins in the spine: a review of current research. Yale J Biol Med 2014;87:549-61. [PubMed]
- Devine JG, Dettori J, France J, et al. The use of rhBMP in spine surgery: is there a cancer risk? Evid Based Spine Care J 2012;3:35-41. [Crossref] [PubMed]
- Chrastil J, Low JB, Whang PG, et al. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine (Phila Pa 1976) 2013;38:E1020-7. [Crossref] [PubMed]
- Singh K, Ahmadinia K, Park DK, et al. Complications of spinal fusion with utilization of bone morphogenetic protein: A systematic review of the literature. Spine (Phila Pa 1976) 2014;39:91-101. [Crossref] [PubMed]
- Faundez A, Tournier C, Garcia M, et al. Bone morphogenetic protein use in spine surgery—complications and outcomes: a systematic review. Int Orthop 2016;40:1309-19. [Crossref] [PubMed]
- James AW, LaChaud G, Shen J, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev 2016;22:284-97. [Crossref] [PubMed]
- Agarwal R, Williams K, Umscheid CA, et al. Osteoinductive bone graft substitutes for lumbar fusion: A systematic review - Clinical article. J Neurosurg Spine 2009;11:729-40. [Crossref] [PubMed]
- Lao L, Cohen JR, Buser Z, et al. Trends Analysis of rhBMP2 Utilization in Single-Level Anterior Lumbar Interbody Fusion in the United States. Global Spine J 2018;8:137-41. [Crossref] [PubMed]
- Lao L, Cohen JR, Buser Z, et al. Trends Analysis of rhBMP Utilization in Single-Level Posterior Lumbar Interbody Fusion in the United States. Global Spine J 2017;7:624-8. [Crossref] [PubMed]
- Yoon ST, Boden SD. Spine fusion by gene therapy. Gene Ther 2004;11:360-7. [Crossref] [PubMed]
- Alden TD, Beres EJ, Laurent JS, et al. The use of bone morphogenetic protein gene therapy in craniofacial bone repair. J Craniofac Surg 2000;11:24-30. [Crossref] [PubMed]
- Brown SJ, Turner SA, Balain BS, et al. Is Osteogenic Differentiation of Human Nucleus Pulposus Cells a Possibility for Biological Spinal Fusion? Cartilage 2020;11:181-91. [Crossref] [PubMed]
- Kloen P, Lauzier D, Hamdy RC. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 2012;51:59-68. [Crossref] [PubMed]
- May RD, Frauchiger DA, Albers CE, et al. Application of Cytokines of the Bone Morphogenetic Protein (BMP) Family in Spinal Fusion - Effects on the Bone, Intervertebral Discs, and Mesenchymal Stromal Cells. Curr Stem Cell Res Ther 2019;14:618-43. [Crossref] [PubMed]
- Poeran J, Opperer M, Rasul R, et al. Change in Off-Label Use of Bone Morphogenetic Protein in Spine Surgery and Associations with Adverse Outcome. Global Spine J 2016;6:650-9. [Crossref] [PubMed]
- Lykissas M, Gkiatas I. Use of recombinant human bone morphogenetic protein-2 in spine surgery. World J Orthop 2017;8:531-5. [Crossref] [PubMed]
- Alt V, Chhabra A, Franke J, et al. An economic analysis of using rhBMP-2 for lumbar fusion in Germany, France and UK from a societal perspective. Eur Spine J 2009;18:800-6. [Crossref] [PubMed]
- Alt V, Haas H, Rauschmann MA, et al. Health-economic considerations for the use of BMP-2 for spinal surgery in Germany. Z Orthop Ihre Grenzgeb 2006;144:577-82. [Crossref] [PubMed]