
Minimally invasive lateral approaches for the treatment of spinal tumors: single-position surgery without the “flip”
Introduction
Although primary cancers of the spine are rare, metastatic presentation is quite common. The most frequent location for osseous metastasis is the spine (1). It has been reported that 30–90% of patients who suffer an oncologic expiration have evidence of spinal metastatic disease (1-3). However, the vast majority of these patients remain asymptomatic. Only 5–40% of cases result in spinal cord compression and less than 10–20% of those require surgical intervention (2). As the population continues to age, the incidence of cancer diagnoses is expected to rise and effective treatments will be increasingly needed (3,4).
Mechanical instability, neurologic symptoms with evidence of spinal cord compression, and refractory pain are the best-accepted indications for surgery (5). Other indications for surgical intervention include local control of tumor burden, and correction or prevention of deformities (1-4). However, there are many factors that affect surgical decision making. The tumor-specific factors include the biologic tumor type, tumor sensitivity to radiation/chemotherapy, evidence of spinal cord compression, and the size/location of the tumor.
Host factors include prior treatments, severity of symptoms, ambulation status, presence of neurologic deficit, general medical condition, treatment expectations, extent of disease, presence of multiple lesions, and life expectancy (1,3,6,7). Overall health of the patient is a major consideration when evaluating their ability to tolerate surgical intervention. Thus, a multidisciplinary approach is helpful to individualize care for each patient and improve outcome. The physician treatment team may include a combination of surgeons (neurosurgery, spine surgery); non-operative physicians (medical oncology, neurology, pathology, medicine, physiatry, palliative care); and procedural physicians (radiation oncology, interventional radiology, pain management) (8).
Despite the requirement for multidisciplinary care for optimal outcome, there remains a lack of universal terminology which complicates evidence-based medicine (EBM) decision-making (6,9,10). Many of the improperly used terms relate to the manner in which the tumor is removed and in the description of tumor margins (9). Inconsistency in reporting of surgical approach and margin prevents accurate comparison between treatment modalities. Spine surgical margins should be described as: wide (removing the entire tumor with a circumferential margin of non-neoplastic tissue); marginal (dissecting through the pseudocapsule of the tumor); or intralesional (neoplastic margins with retained tumor). Additionally, standard tumor resection naming should include the terms piecemeal (where the tumor is removed in pieces) or en bloc (where the tumor is removed as a whole) (8,9,11-16). It is critical to accurately describe resection margins as it may have a direct effect on survival and risk of recurrence (9).
History
Surgery for spinal tumors is a relatively recent advancement in the treatment of cancer patients. Traditionally, particularly in the years prior to 1980, radiation therapy was considered the primary and sole treatment modality for spinal tumors (10,17,18). Surgery was often palliative in nature with laminectomies reserved for epidural metastasis (10,17-19). In 1980, a landmark randomized trial comparing laminectomy followed by radiotherapy to radiotherapy alone reported no difference in patient outcome, but increased morbidity in the surgical group (19). Despite subsequent reports suggesting improved function and survivability with ventral decompression, avoidance of surgical intervention for spinal tumors perpetuated through the early 1990s (20-25). With advancements in surgical techniques and EBM, however, there has been a shift from surgical intervention being primarily palliative—which it remains in most cases—to potentially curative (6).
In 2005, Patchell and colleagues published the most influential randomized, controlled trial for spinal tumors to-date. The study was terminated early and published in Lancet due to the significant disparity in outcomes between the two treatment groups. In the series of 101 patients, direct decompressive surgery followed by radiation significantly improved outcome over radiation alone. The surgical group ambulated at a higher percentage (84% vs. 57%) for a longer period of time (62% vs. 19%) with less steroid and opioid requirements. From this study, the Patchell criteria for surgical intervention have been extrapolated: (I) neurologic deficit, including refractory pain; (II) not exquisitely radiosensitive (i.e., lymphoma, plasmacytoma); (III) radiographic evidence of spinal cord compression; and (IV) life expectancy of at least 3 months (20). A meta-analysis of surgery versus radiotherapy confirmed the findings of Patchell reporting that surgical patients were twice as likely to regain ambulatory function. Overall, ambulation was achieved in 85% of surgical patients and 64% of radiation patients (26).
Oncologic patients often suffer significant comorbidities, including a compromised immune system and poor protoplasm. Suppressed immune function and a history of radiation therapy has been correlated with higher risks of complications, local recurrence, and wound breakdown in open, posterior-based surgery (27-32). The development of minimally invasive (MIS) approaches may mitigate these untoward complications (33-52).
Determining surgical approach
After operative intervention has been indicated, the surgeon must preoperatively plan the optimal surgical approach to adequately address the pathology while minimizing potential complications. In extremity tumors, surgical staging may assist in the decision-making process, however, standard oncological staging systems are not applicable to spine tumors (6,9,44). The most accepted classification systems for spine tumors are the Enneking staging system (53,54) and the Weinstein, Boriani, and Bagini (WBB) classification system (55).
Using the WBB classification scheme, the axial spine is divided into 12 equal parts. A five-layer classification is used to show infiltration of the tumor from the paravertebral zone to within the dura (Figure 1). The WBB system may guide surgical decision-making based on the zone of the lesion, with corpectomies for zones 4–8 and 5–9, sagittal resections for zones 2–5 and 7–11, and posterior-based approaches for tumors localized to zones 10–3 (9,55).

The location, morphology, and tumor biology must also be considered in the process of preoperative planning. Over 65% of tumors affect the vertebral body and anterior column with less than 33% isolated to the posterior arch (1). Based solely on location alone, anterior exposures have become the “gold standard” approach given their superior access to the anteriorly-based pathology (8,16,44,45,47-49,56). The utilization of posterior-based approaches for anterior-based tumors requires excessive bony resection of the posterior elements necessitating multi-level stabilization (1,45). However, given most surgeons’ familiarity and comfort with the approach, posterior-based treatment for spinal tumors remains a conventional technique. However, MIS posterior approaches to anterior corpectomy have reported utility in treating tumors involving both the anterior and posterior columns (36,57).
The most common location for metastatic spinal lesions is the thoracic (70%) and lumbar (20%) spine, followed by the cervical (10%) spine (3). In general, the respective anatomical considerations of each area of the spine dictate potential surgical approach. For example, posterior approaches are most commonly utilized in the upper cervical spine (39), while anterior approaches are favored in the subaxial cervical spine. Combined anterior and posterior approaches may be employed based on host bone quality and extent of instability after tumor resection (39). In the upper thoracic spine extending to T5, anterior approaches are complicated by the great vessels; however, when necessary, a trap-door or conventional sternotomy may allow adequate access (Figure 2).
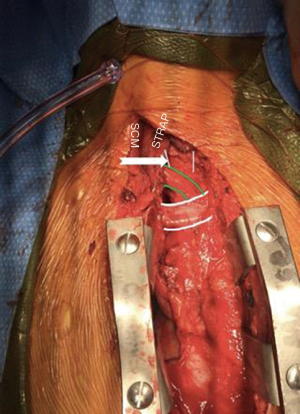
Conventional surgical techniques
Holman and colleagues reported on patients with lumbar metastatic disease treated with a posterior decompression and posterolateral fusion, transpedicular vertebrectomy, or combined anterior-posterior approaches (28). Anterior vertebrectomy resulted in lower blood loss when compared to transpedicular approaches (1,375 vs. 2,000 cc). There was a complete absence of infections after anterior procedures, compared with 11% after posterior procedures. The highest overall complication rate was found in combined, staged anterior-posterior approaches (75%) (28). Anterior and combined anterior-posterior procedures resulted in higher rates of neurologic improvement when compared to posterior approaches alone (41% vs. 50% vs. 27%, respectively).
A similar study evaluated simultaneous anterior-posterior approaches for treatment of 26 patients diagnosed with complex thoracolumbar spinal metastases. Mean operative time was 636 min (range, 423–882 min) with a median blood loss of 2,100 cc (range, 750–10,000 cc). Median length of stay (LOS) was 10.5 days (range, 4–57 days). Nine (35%) major early complications occurred in seven (27%) patients, including cases of an inadvertent durotomy and subsequent meningitis, deep wound infection, and neurological deterioration. The overall incidence of complications was 54%. Neurologic status was maintained or improvement in 96.2% of patients, and one-year survival was reported at 68% (58).
In a series of 26 patients diagnosed with spinal malignancies treated with en bloc resection, Fisher and colleagues reported wide surgical margins achieved in 15 patients, marginal in 4 patients, and intralesional in 7 patients. Mean operative time (including staging) was 18.6 h (range, 1.3–56.3 h) with an average blood loss of 3,880 cc. Blood loss was classified as “massive” in 42.3% of patients (>5,000 cc). Complications were reported in 92% of patients with 15.4% attributed to infection (9).
In a series of open, transpedicular approaches for treatment of spinal metastases with spondylectomy, decompression, and circumferential fusion, Bilsky et al. reported a complication rate of 48%, including two infections and three 30-day mortalities (59). Another study of 67 patients who underwent posterior decompression and fusion for spinal tumors reported a 19.4% wound complication and 16% infection rate (60). Similarly, Harrington reported a 50% incidence of infection after posterior procedures for compared to 1.3% in anterior procedures (61). Overall, the literature on conventional surgical treatment of spinal tumors suggests significantly increased rates of postoperative infection following open, posterior approaches.
MIS techniques
There has been a rapid expansion in the utilization of MIS techniques in spine surgery over the past decade. Advancements in instrumentation, retractors, and surgical guidance have provided surgeons with muscle-sparing approaches for adequately addressing predominantly degenerative pathology. However, MIS techniques have been recently developed for the treatment of tumor pathology in the thoracolumbar spine.
Single-position surgery employs a mini-open lateral approach, which allows concurrent access to the anterior and posterior columns of the spine for tumor resection and corpectomy (36,51,57,62-67). The technique is a relatively recent advancement in MIS spine surgery introduced to mitigate the complications of open, posterior procedures, while still achieving equivalent or improved patient outcome. Fixation options include lateral plating, transpsoas screw-rod construct, and percutaneous posterior pedicle screws instrumented with the patient in the lateral decubitus position. Stabilization is obtained without having to stage the surgery or intraoperatively reposition, or “flip”, the patient, which may have the additional benefit of avoiding the cardiopulmonary complications associated with prolonged, prone anesthesia.
Mini-open lateral transpsoas approach
The mini-open, lateral retroperitoneal transpsoas approach for lumbar interbody fusion (LLIF) was first reported in the literature in 2006 (68). The lateral transpsoas approach has been expanded to access cephalad levels to T5/6, allowing for “gold standard” anterior exposure while minimizing the associated morbidity associated with traditional thoracotomies (69).
The mini-open approach utilizes blunt dissection through the retroperitoneal space and psoas muscle in the lumbar spine, or through the retropleural/transpleural space in the thoracic spine, to access the lateral spine for ventral decompression of the spinal canal. Advancements in intraoperative neuromonitoring have been integral to the success of the procedure, particularly in the lumbar spine, by permitting real-time discrete and directional electromyographic data and allowing safe navigation around the lumbar plexus (70). The utility of the procedure has been well described for degenerative conditions (69,71-76), but significantly less reported for spinal tumor indications (51,66,73).
Small case series on this surgical approach have shown promising results. In a series of three patients treated with the LLIF approach for neurofibroma removal, Dakwar et al. reported a mean blood loss of 150 mL, operative time of 85 min, and LOS of 2 days. There were no complications, and patients achieved significant improvement in pain and function (66). Similarly, a case report of a neurofibroma located in the T11–T12 neural foramen treated with a retropleural approach and LLIF reported blood loss of 150 cc and operative time of 2 hours without complication (73).
In 2010, a study was published on 21 consecutive patients with an average of 21 months follow-up after treatment with the LLIF approach for thoracic tumors. The most common tumor type was meningioma, followed by neurofibroma, and plasmacytoma. Thirteen patients underwent anterior corpectomy (62%), 5 underwent interbody fusion (23%), and the remainder were left uninstrumented (15%). Instrumentation included anterolateral plating (72%) and pedicle screw fixation (28%). Uribe and colleagues reported significant improvements over traditional, open surgical techniques, with a mean blood loss of 291 cc and LOS of 3 days. There was a 62% and 53% improvement in patient reported outcomes, visual analog scale (VAS) and Oswestry disability index (ODI), respectively. The only postoperative complication was pneumonia (5%) (51).
Author’s preferred surgical technique
Lumbar corpectomy
Corpectomy in the lumbar spine is a direct extension of the LLIF technique. The retroperitoneal space is exposed through a flank incision placed in Langer’s lines. The lateral border of the psoas muscle is accessed using blunt finger dissection. Under fluoroscopic guidance and real-time, directional neuromonitoring, sequential dilators are employed for access to lateral aspect of the anterior spine. Separate exposures through the psoas muscle are used to first access the disc spaces above and below the corpectomy level to allow for complete discectomies and endplate preparation (Figure 3).
The third exposure through the psoas muscle is mid-vertebral. The segmental artery is identified and ligated or coagulated with bipolar cautery. It is imperative that lateral fluoroscopy is consistently verified for true lateral orientation and orthogonality to the floor to allow safe retractor placement and creation of the working window. The posterior blade of the retractor establishes the working corridor anterior to the dura, while the fourth blade, or anterior blade, is placed over the ALL providing protection from the great vessels.
An Epstein curette may be used for ventral decompression of the dura, as the position of the lumbar plexus may make direct visualization of the thecal sac challenging, particularly in the caudal segments of the lumbar spine. Upon completion of the corpectomy, an expandable vertebral body replacement (VBR) device is placed in the defect. Anterolateral fixation can be used for a single-incision approach (Figure 4), or percutaneous posterior fixation may be placed with fluoroscopic guidance.

Corpectomy at the thoracolumbar junction
The MIS, single-position lateral approach at the thoracolumbar junction requires the anatomic consideration of the diaphragm and the pleural cavity. It is important to prevent violation of the diaphragm and the pleura during the approach to minimize the risk of debris polluting the lung, cancer spread, and intrapleural complications. Gentle retraction and mobilization of the diaphragm along natural tissue planes limits violation and potentially, lowers iatrogenic morbidity. If the pleura are violated during the procedure, a chest tube should be placed postoperatively. Additionally, if the diaphragm is violated, repair is generally not necessary if the defect is less than 2 cm.
Typically, resection of a portion of the T11 rib is required for the approach to thoracolumbar corpectomy. After rib resection, digital dissection begins along the T12 rib to mobilize its associated neurovascular bundle. The diaphragm is then mobilized medially and superiorly to develop a communication between the retropleural and retroperitoneal space. The retractor is used to access the lateral thoracic spine in a retropleural fashion. Retractor placement is critical, with careful attention to dock it far enough posterior to allow access to the ipsilateral lamina, facet, and pedicle. Often, the posterior blade of the retractor is 10–20 mm shorter to account for docking on the rib head. The corpectomy procedure is performed in standard fashion (Figure 5).
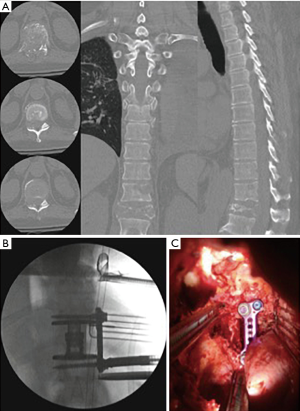
Thoracic corpectomy
With experience, it is possible to perform a retropleural exposure up to the T6 or T7 levels. Again, a well-dissected retropleural approach reduces pulmonary complications and limits seeding of tumor into the lung. Unfortunately, in patients with significant systemic illness and poor overall health, the pleura may be fragile and a transthoracic approach to the lateral spine may be required. If required, a laparotomy sponge can be placed on the border between the lung and the retractor blade for further protection of the lung during the procedure. By not requiring a dual-lumen intubation, the MIS lateral approach in the thoracic spine may reduce the risk of atelectasis and pneumonia postoperatively.
In lateral thoracic approaches, preoperative CT evaluation of the level to be treated is critical to understand the position of the rib head with respect to the canal, the disc, and the pedicle. Once docked on the lateral aspect of the thoracic spine, the rib head can be excised using rongeurs, a high-speed drill, or osteotome based on surgeon preference. Once the rib articulation is removed, a pediculectomy is performed under direct fluoroscopic guidance, allowing exposure and direct visualization of the spinal canal and dura. Using intraoperative AP fluoroscopy, the high-speed drill can be used to thin down the pedicle in lateral-to-medial direction, and the medial cortical layer of the pedicle can be removed at the end of this maneuver using a Kerrison punch or an equivalent instrument. The corpectomy procedure proceeds in standard fashion.
Conclusions
Over the last 20 years, there have been significant advancements in the surgical treatment of spinal tumors, including the development of MIS, single-position lateral approaches. The management of spinal tumors to the spine is continually changing and the shifting paradigm of metastatic disease may reflect a modified role for surgical intervention (77,78). As our interventions become less morbid, the surgical indications must not change. Still, surgical approach is dictated by the tumor histology, patient prognosis, spinal stability, neurologic impairment, general patient health, and patient preference. Therefore, operative treatment of tumors in the future may be a consolidation of historical surgical techniques and MIS, single-position lateral approaches. Regardless, multidisciplinary management is imperative for the individualized treatment of the patient and optimization of outcome.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Dr. Laratta reports personal fees from Stryker/K2m, NuVasive, Spineart, Evolution Spine, American Institute of Minimally Invasive Surgery, and Gerson Lehrman Group; grants from Medtronic, NuVasive, Orthopaedic Science Research Foundation, and Fisher-Owen Fund; and Editorial Board of the following: Spine, Global Spine Journal, and Journal of Spine Surgery. Dr. Smith reports personal fees from NuVasive, Spineology, Pediguard, Providence, and Ortho Bio Design; as well as grants from NuVasive, Providence, Spineology, and Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bell GR. Surgical treatment of spinal tumors. Clin Orthop Relat Res 1997.54-63. [Crossref] [PubMed]
- Klimo P Jr, Kestle JR, Schmidt MH. Treatment of metastatic spinal epidural disease: a review of the literature. Neurosurg Focus 2003;15:E1. [Crossref] [PubMed]
- Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. J Neurosurg Spine 2010;13:94-108. [Crossref] [PubMed]
- Gokaslan ZL. Spine surgery for cancer. Curr Opin Oncol 1996;8:178-81. [Crossref] [PubMed]
- Csaszar N, Ganju A, Mirnics ZS, et al. Psychosocial issues in the cancer patient. Spine 2009;34:S26-30. [Crossref] [PubMed]
- Fisher CG, Andersson GB, Weinstein JN. Spine focus issue. Summary of management recommendations in spine oncology. Spine 2009;34:S2-6. [Crossref] [PubMed]
- Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine 1997;22:1773-82. [Crossref] [PubMed]
- Delank KS, Wendtner C, Eich HT, et al. The treatment of spinal metastases. Dtsch Arztebl Int 2011;108:71-9. [PubMed]
- Fisher CG, Keynan O, Boyd MC, et al. The surgical management of primary tumors of the spine: initial results of an ongoing prospective cohort study. Spine 2005;30:1899-908. [Crossref] [PubMed]
- Fisher CG, Keynan O, Ondra S, et al. Introduction to focus issue in spine oncology: the synthesis of evidence and expert opinion for best practice recommendation. Spine 2009;34:S21-5. [Crossref] [PubMed]
- Andersson GB, Chapman JR, Dekutoski MB, et al. Do no harm: the balance of “beneficence” and “non-maleficence”. Spine 2010;35:S2-8. [Crossref] [PubMed]
- Dekutoski MB, Norvell DC, Dettori JR, et al. Surgeon perceptions and reported complications in spine surgery. Spine 2010;35:S9-21. [Crossref] [PubMed]
- Oner FC, Ramos LM, Simmermacher RK, et al. Classification of thoracic and lumbar spine fractures: problems of reproducibility. A study of 53 patients using CT and MRI. Eur Spine J 2002;11:235-45. [Crossref] [PubMed]
- Vaccaro AR, Kim DH, Brodke DS, et al. Diagnosis and management of thoracolumbar spine fractures. Instr Course Lect 2004;53:359-73. [PubMed]
- Vaccaro AR, Lim MR, Hurlbert RJ, et al. Surgical decision making for unstable thoracolumbar spine injuries: results of a consensus panel review by the Spine Trauma Study Group. J Spinal Disord Tech 2006;19:1-10. [Crossref] [PubMed]
- Cherqui A, Kim DH, Kim SH, et al. Surgical approaches to paraspinal nerve sheath tumors. Neurosurg Focus 2007;22:E9. [Crossref] [PubMed]
- Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery 1979;5:726-46. [Crossref] [PubMed]
- Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg 1983;59:111-8. [Crossref] [PubMed]
- Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg 1980;53:741-8. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Siegal T, Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: a prospective study. Neurosurgery 1985;17:424-32. [Crossref] [PubMed]
- Siegal T, Tiqva P, Siegal T. Vertebral body resection for epidural compression by malignant tumors. Results of forty-seven consecutive operative procedures. J Bone Joint Surg Am 1985;67:375-82. [Crossref] [PubMed]
- Siegal T, Siegal T. Treatment of malignant epidural cord and cauda equina compression. Prog Exp Tumor Res 1985;29:225-34. [PubMed]
- Falicov A, Fisher CG, Sparkes J, et al. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine 2006;31:2849-56. [Crossref] [PubMed]
- Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998;89:599-609. [Crossref] [PubMed]
- Klimo P Jr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76. [Crossref] [PubMed]
- Polly DW Jr, Chou D, Sembrano JN, et al. An analysis of decision making and treatment in thoracolumbar metastases. Spine 2009;34:S118-27. [Crossref] [PubMed]
- Holman PJ, Suki D, McCutcheon I, et al. Surgical management of metastatic disease of the lumbar spine: experience with 139 patients. J Neurosurg Spine 2005;2:550-63. [Crossref] [PubMed]
- Bohinski RJ, Rhines LD. Principles and techniques of en bloc vertebrectomy for bone tumors of the thoracolumbar spine: an overview. Neurosurg Focus 2003;15:E7. [Crossref] [PubMed]
- Riaz S, Fox R, Lavoie MV, et al. Vertebral body reconstruction for thoracolumbar spinal metastasis—a review of techniques. J Ayub Med Coll Abbottabad 2006;18:70-7. [PubMed]
- Wai EK, Finkelstein JA, Tangente RP, et al. Quality of life in surgical treatment of metastatic spine disease. Spine 2003;28:508-12. [Crossref] [PubMed]
- Sundaresan N, Rothman A, Manhart K, et al. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine 2002;27:1802-6. [Crossref] [PubMed]
- Hsieh PC, Koski TR, Sciubba DM, et al. Maximizing the potential of minimally invasive spine surgery in complex spinal disorders. Neurosurg Focus 2008;25:E19. [Crossref] [PubMed]
- Huang TJ, Hsu RW, Li YY, et al. Minimal access spinal surgery (MASS) in treating thoracic spine metastasis. Spine 2006;31:1860-3. [Crossref] [PubMed]
- Krisht KM, Mumert ML, Schmidt MH. Management considerations and strategies to avoid complications associated with the thoracoscopic approach for corpectomy. Neurosurg Focus 2011;31:E14. [Crossref] [PubMed]
- Lu DC, Chou D, Mummaneni PV. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors. J Neurosurg Spine 2011;14:758-64. [Crossref] [PubMed]
- O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 2009;11:471-6. [Crossref] [PubMed]
- Parker SL, Adogwa O, Witham TF, et al. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg 2011;54:33-7. [Crossref] [PubMed]
- Fehlings MG, David KS, Vialle L, et al. Decision making in the surgical treatment of cervical spine metastases. Spine 2009;34:S108-17. [Crossref] [PubMed]
- Yin QS, Ai FZ, Zhang K, et al. Transoral atlantoaxial reduction plate internal fixation for the treatment of irreducible atlantoaxial dislocation: a 2- to 4-year follow-up. Orthop Surg 2010;2:149-55. [Crossref] [PubMed]
- Yin QS, Ai FZ, Zhang K, et al. Transoral atlantoaxial reduction plate fixation for irreducible atlantoaxial dislocation. Chin J Traumatol 2006;9:14-20. [PubMed]
- Yin Q, Ai F, Zhang K, et al. Irreducible anterior atlantoaxial dislocation: one-stage treatment with a transoral atlantoaxial reduction plate fixation and fusion. Report of 5 cases and review of the literature. Spine 2005;30:E375-81. [Crossref] [PubMed]
- Amini A, Beisse R, Schmidt MH. Thoracoscopic spine surgery for decompression and stabilization of the anterolateral thoracolumbar spine. Neurosurg Focus 2005;19:E4. [Crossref] [PubMed]
- Boriani S, Biagini R, De IF, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine 1996;21:1927-31. [Crossref] [PubMed]
- Hall DJ, Webb JK. Anterior plate fixation in spine tumor surgery. Indications, technique, and results. Spine 1991;16:S80-3. [Crossref] [PubMed]
- Kan P, Schmidt MH. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus 2008;25:E8. [Crossref] [PubMed]
- King GJ, Kostuik JP, McBroom RJ, et al. Surgical management of metastatic renal carcinoma of the spine. Spine 1991;16:265-71. [Crossref] [PubMed]
- Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation results. Spine 1983;8:512-31. [Crossref] [PubMed]
- Lewandrowski KU, Hecht AC, DeLaney TF, et al. Anterior spinal arthrodesis with structural cortical allografts and instrumentation for spine tumor surgery. Spine 2004;29:1150-8. [Crossref] [PubMed]
- Sakaura H, Hosono N, Mukai Y, et al. Outcome of total en bloc spondylectomy for solitary metastasis of the thoracolumbar spine. J Spinal Disord Tech 2004;17:297-300. [Crossref] [PubMed]
- Uribe JS, Dakwar E, Le TV, et al. Minimally invasive surgery treatment for thoracic spine tumor removal: a mini-open, lateral approach. Spine 2010;35:S347-54. [Crossref] [PubMed]
- Yao KC, Boriani S, Gokaslan ZL, et al. En bloc spondylectomy for spinal metastases: a review of techniques. Neurosurg Focus 2003;15:E6. [Crossref] [PubMed]
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980.106-20. [PubMed]
- Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986.9-24. [PubMed]
- Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine 1997;22:1036-44. [Crossref] [PubMed]
- Heary RF, Bono CM. Metastatic spinal tumors. Neurosurg Focus 2001;11:e1. [Crossref] [PubMed]
- Lu DC, Lau D, Lee JG, et al. The transpedicular approach compared with the anterior approach: an analysis of 80 thoracolumbar corpectomies. J Neurosurg Spine 2010;12:583-91. [Crossref] [PubMed]
- Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg 2001;94:232-44. [PubMed]
- Bilsky MH, Boland P, Lis E, et al. Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine 2000;25:2240-9; discussion 2250. [Crossref] [PubMed]
- Bauer HC. Posterior decompression and stabilization for spinal metastases. Analysis of sixty-seven consecutive patients. J Bone Joint Surg Am 1997;79:514-22. [Crossref] [PubMed]
- Harrington KD. Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop Relat Res 1988.177-97. [PubMed]
- Lucio JC, VanConia RB, Deluzio KJ, et al. Economics of less invasive spinal surgery: an analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy 2012;5:65. [PubMed]
- Smith WD, Christian G, Serrano S, et al. A comparison of perioperative charges and outcome between open and mini-open approaches for anterior lumbar discectomy and fusion. J Clin Neurosci 2012;19:673-80. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Rodgers JA. Lumbar fusion in octogenarians: the promise of minimally invasive surgery. Spine 2010;35:S355. [Crossref] [PubMed]
- Chou D, Lu DC. Mini-open transpedicular corpectomies with expandable cage reconstruction. Technical note. J Neurosurg Spine 2011;14:71-7. [Crossref] [PubMed]
- Dakwar E, Smith WD, Malone KT, et al. Minimally invasive lateral extracavitary resection of foraminal neurofibromas. J Clin Neurosci 2011;18:1510-2. [Crossref] [PubMed]
- Lu DC, Dhall SS, Mummaneni PV. Mini-open removal of extradural foraminal tumors of the lumbar spine. J Neurosurg Spine 2009;10:46-50. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Smith WD, Dakwar E, Le TV, et al. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine 2010;35:S338-46. [Crossref] [PubMed]
- Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine 2011;14:31-7. [Crossref] [PubMed]
- Baaj AA, Dakwar E, Le TV, et al. Complications of the mini-open anterolateral approach to the thoracolumbar spine. J Clin Neurosci 2012;19:1265-7. [Crossref] [PubMed]
- Khan SN, Cha T, Hoskins JA, et al. Minimally invasive thoracolumbar corpectomy and reconstruction. Orthopedics 2012;35:e74-9. [PubMed]
- Uribe JS, Dakwar E, Cardona RF, et al. Minimally invasive lateral retropleural thoracolumbar approach: cadaveric feasibility study and report of 4 clinical cases. Neurosurgery 2011;68:32-9. [PubMed]
- Deviren V, Kuelling FA, Poulter G, et al. Minimal invasive anterolateral transthoracic transpleural approach: a novel technique for thoracic disc herniation. A review of the literature, description of a new surgical technique and experience with first 12 consecutive patients. J Spinal Disord Tech 2011;24:E40-8. [Crossref] [PubMed]
- Karikari IO, Nimjee SM, Hardin CA, et al. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: initial clinical experience and early outcomes. J Spinal Disord Tech 2011;24:368. [Crossref] [PubMed]
- Uribe JS, Smith WD, Pimenta L, et al. Minimally invasive lateral approach for symptomatic thoracic disc herniation: initial multicenter clinical experience. J Neurosurg Spine 2012;16:264-79. [Crossref] [PubMed]
- Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys 2008;71:652-65. [Crossref] [PubMed]
- Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 2011;14:151-66. [Crossref] [PubMed]


