
Treatment of degenerative spondylolisthesis by instrumented posterolateral versus instrumented posterolateral with transforaminal lumbar interbody single-level fusion
Introduction
Degenerative spondylolisthesis is an acquired subluxation of one vertebrate relative to an adjacent vertebrate commonly resulting in spinal stenosis (1). Försth et al. studied the effectiveness decompression surgery with or without instrumented fusion in stenosis patients with or without degenerative spondylolisthesis (2). Decompression plus fusion did not result in better clinical outcomes compared to decompression alone at 2 and 5 years post-operatively. This was true for patients with degenerative spondylolisthesis as well as those without. Alternatively, when Ghogawala et al. studied the efficacy of decompression surgery with or without instrumented fusion (pedicle screws and rods) in stenosis patients with grade 1 lumbar degenerative spondylolisthesis, they found greater and clinically meaningful improvement in health-related quality of life for decompression plus fusion compared to decompression alone at 2, 3, and 4 years post-operatively (3). Likewise, the Spine Patient Outcomes Research Trial (SPORT) found surgical treatment is beneficial over non-operative measures at both two- and four-year intervals (4-6). However, this trial included treatments ranging from decompression alone to 360° fusion. For example, sub-group analysis of the SPORT outcomes did not show a difference between posterolateral in situ lumbar fusion (PLF), posterolateral fusion with pedicle screws, or posterolateral fusion with pedicle screws and interbody fusion at four years (7). The optimal surgical intervention remains, therefore, debatable.
Harms and Rolinger first described transforaminal lumbar interbody fusion (TLIF) in 1982 (8). Many studies have addressed whether TLIF adds benefit to PLF for spondylolisthesis (9-16). While some studies have found benefits to posterior interbody fusion, others have found no difference between anterior and posterior procedures. This may be because the outcomes in some studies (for example, the sub-group analysis of SPORT outcomes by Abdu et al.) grouped surgical procedures such as anterior and posterior interbody fusions together (7). Alternatively, this may be because of grouping diagnoses together. For example, most group isthmic and degenerative spondylolisthesis together though these are two different disease processes (17).
Given the lack of consensus on treatment, we retrospectively analyzed the functional outcome between instrumented posterolateral lumbar fusion alone (PLF) and PLF with TLIF (PLF + TLIF) in the treatment of low-grade degenerative spondylolisthesis at two-year follow-up. We also studied the co-morbidities, surgical data, complications, costs, and acute reoperation rates.
Methods
Inclusion and exclusion criteria
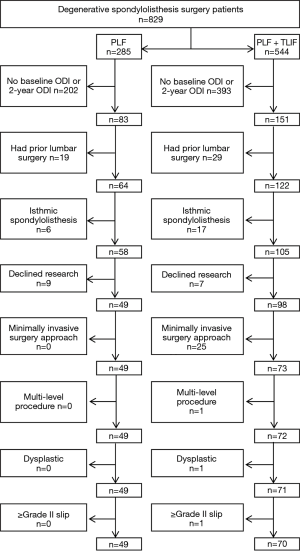
Institutional approval for this study was obtained through Quorum Review IRB. After IRB approval, a query of all patients treated for degenerative spondylolisthesis (ICD-9 738.4) between January 2009 and December 2011 with PLF or PLF + TLIF at our institution was made. Patients were included if they had had an open single-level fusion and if pre-operative and 2-year post-operative Oswestry Disability Index (ODI) were documented. Patients were excluded if they had had prior lumbar surgery, had a high grade slip (grade 3 or above), were found to have an isthmic spondylolisthesis, had declined participation in research review, or were minors.
Operative technique
All patients were positioned prone and a standard, open midline dissection was performed. There were seven surgeons included in the study and access to spinal canal was performed either through laminectomy or hemilaminotomy, the choice being that of the surgeon. All patients received central and/or foraminal decompression at one or more levels and pedicle screw instrumentation or pedicle screw instrumentation plus an interbody spacer at one level. In the PLF group, intertransverse fusion was performed bilaterally, while in the PLF + TLIF group it was performed on the side contralateral to the facetectomy performed for the TLIF. Bone graft included local bone supplemented by fresh frozen allograft; in some cases, recombinant human bone morphogenetic protein (rhBMP-2) was used. Patients were asked to wear a lumbar brace for 3 months post-operatively.
Clinical measures of results
Clinic and hospital records were reviewed for demographic data [age, body mass index (BMI), smoking status, physical fitness], surgical information (estimated blood loss, procedure (hemilaminotomy versus laminectomy, rhBMP-2 use), complications (dural tears, epidural hematoma, infection), cost (total operating room time, length of hospital stay, implant cost (what a patient’s insurance paid), BMP cost), and acute reoperation status for three years post-operatively, and functional status [ODI, American Society of Anesthesiologists (ASA) (18)].
Statistical analyses
Student’s t-test was used to compare the continuous data and the Chi-square test was used to compare the categorical data between PLF and PLF + TLIF. Patient demographics, pre-operative functional status (ODI), surgical data, peri-operative complications, and costs were compared between PLF and PLF + TLIF. Numbers of levels decompressed were compared by the Mann-Whitney rank sum test as were changes in ODI status at two-year follow-up.
Results
Patient demographics and pre-operative status
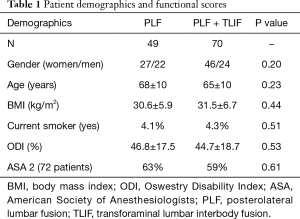
A total of 829 patients were treated for degenerative spondylolisthesis between January 2009 and December 2011 with PLF (n=285) or PLF + TLIF (n=544) at our institution (Figure 1). Of these, 49 (27 F/22 M) patients met inclusion/exclusion criteria in the PLF group and 70 (46 F/24 M) in the PLF + TLIF group (Table 1). There were no statistically significant differences between the groups in regards to age, BMI, and smoking status. Pre-operative functional (ODI) scores were not statistically different between groups. With regard to physical fitness, the PLF and PLF + TLIF groups were not different in ASA status (16): among PLF patients, 63% were ASA 2 status and 37% were ASA 3; among PLF + TLIF patients, 59% were ASA 2 and 41% were ASA 3.
Full table
Surgical observations
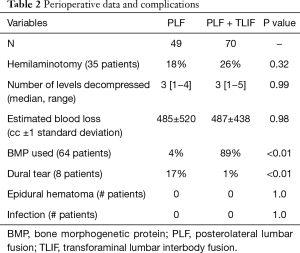
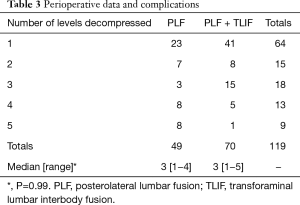
Perioperative surgical data can be found in Table 2. All patients were decompressed and fused at one level and decompressed at up to four additional levels (Table 3); there was no difference between groups in the number of levels decompressed. There was no difference in blood loss. Recombinant human bone morphogenetic protein (rhBMP-2) usage was significantly different between the two groups (P<0.01), being used in 89% of PLF + TLIF procedures and 4% of PLF procedures. Dural tears occurred more commonly in the PLF cohort. There was no statistical difference in epidural hematoma or infections requiring a return to the operating room.
Full table
Full table
Costs
The operative time was significantly shorter and instrumentation cost was less in the PLF cohort (Table 4) [note that the cost of the bone morphogenetic protein (BMP) was excluded from the analyses because including its cost would have substantially favored the PLF group, regardless of other costs]. Reoperations performed through 2014 were tracked and there was no statistical difference between the groups.
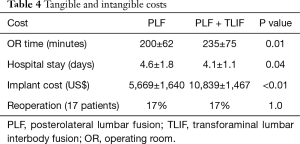
Full table
Clinical outcomes
Post-operative functional (ODI) scores were not statistically different between groups (Table 5). The ODI improvement (change) at two years from baseline in each group was the same for the two surgical treatments (PLF: 17.9%±19.5%; PLF + TLIF: 17.9%±20.0%). There was no statistically significant difference between the two groups (P=0.97, power =0.05).
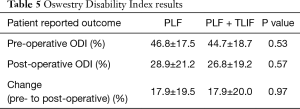
Full table
When all patients were considered together (PLF with PLF + TLIF), current smokers tended to see their ODI scores worsen, while non-smokers tended to see their ODI scores improve (Table 6). This difference was significant (P=0.047). For PLF patients alone or PLF + TLIF patients alone, the differences between current, past, and non-smokers was not significant, even though the trends were similar to those of all patients.
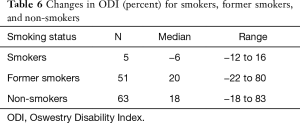
Full table
The effects of health status (ASA), number of levels decompressed, hemilaminectomy versus laminotomy, and fhBMP-2 use on functional outcome (change in ODI score) was similarly studied, but no statistically signification differences were found between the PLF and PLF + TLIF groups.
Discussion
Our results fail to show any statistically significant or clinically significant difference in the functional outcomes as measured by the ODI between PLF and PLF + TLIF cohorts at two-year follow-up (P=0.97). Sixty-three percent of the patients in the PLF group and 61% in the PLF + TLIF group had a clinically significant ODI improvement of 12.8 points or more (19). ODI improvement for both groups were similar to that reported by others (20,21). In the PLF group 20% had worsening of their ODI from pre-op versus 19% in the PLF + TLIF group. In addition, we found no statistical differences in reoperation rate, infection, or epidural hematomas. Hospital stay was slightly shorter for the PLF + TLIF group, but the PLF cohort had shorter OR time and significantly lower implant cost compared to PLF + TLIF. Recombinant human bone morphogenetic protein (rhBMP-2) was used significantly less often in the PLF group compared to the PLF + TLIF procedures.
As noted above, roughly 20% of our patients had worse outcome scores after surgery, Unfortunately, for the individual patient, this outcome is not uncommon. Others have also reported on greater (worse) functional outcomes scores after surgery (3,10,12,13). Our retrospective study was not designed to identify specific causes of failed back surgery for which there is a myriad of reasons, including inadequate a nerve root decompression, inadequate stabilization, failure to fuse, instrumentation failure, and epidural fibrosis (22). Careful screening of patients prior to surgery will help reduce peri- and post-operative risks and contribute to better function outcomes (23).
Our study is one of the largest comparing outcomes of PLF versus PLF + TLIF in only degenerative spondylolisthesis (24). Other studies are heterogeneous, combining degenerative disc disease (DDD), isthmic spondylolisthesis and degenerative spondylolisthesis. In isthmic spondylolisthesis, where foraminal stenosis is a more common pathology, the indirect decompression of the foramen through disc height restoration gives PLF + TLIF a theoretical advantage. PLF + TLIF has a theoretical advantage over PLF in DDD, too, of removing, rather than just stabilizing, the degenerated disc. For this reason, we feel it is important to stratify, rather than group DDD, isthmic spondylolisthesis and degenerative spondylolisthesis.
When comparing PLF to PLF + TLIF, others report mixed results, ranging from equivalence between the two to favoring PLF + TLIF. For degenerative spondylolisthesis alone, we found no difference between PLF and PLF + TLIF in functional outcome. On the other hand, OR time, implant cost, and rhBMP-2 use considerably favor PLF. Results between PLF and PLF + TLIF were not dependent upon smoking status, although current smokers tended to see their ODI scores worsen, while non-smokers tended to see their ODI scores improve.
BMP was used in the large majority of PLF + TLIF cases and two PLF cases. This difference reflects surgeon preferences at the time and the clinical outcomes did not seem to be affected. However, BMP is approved for anterior open or anterior laparoscopic approaches only. There have been reports of ectopic bone formation when BMP has been used during MIS TLIF procedures (25), but this was not our experience.
There was a 17% dural tear rate in the PLF group and 1% in the TLIF group. This difference was statistically significant. The reason for the difference is not known. Perhaps there was less direct and more indirect decompression in the TLIF cases, but this is speculative. Nevertheless, the overall results and clinical outcomes did not seem to be affected. This was similarly observed in the SPORT trial, which concludes: “Incidental durotomy during first time surgery for lumbar degenerative spondylolisthesis does not appear to impact outcome in affected patients” (26). There was no apparent relationship between surgeon and incidences of dural tears as the complication occurred twice for three surgeons, once for three surgeons and never for one surgeon. Others’ reports on this complication include Li et al.: no dural tears among 40 patients (20 PLF and 20 TLIF) (15); Jalalpour et al.: two dural tears among 67 patients receiving uninstrumented PLF (3%) versus zero among 68 patients receiving TLIF (0%) (10); Fujimori et al.: four dural tears (12.5%) with PLF (n=32) and one (4%) with TLIF (n=24) (12); and Christensen et al.: one dural tear among 47 PLF patients (2%) and two among 51 TLIF patients (4%) (13). Al Barbarawi et al. reported no difference in complication rates (including dural tears) between PLF, TLIF and PLIF (posterior lumbar interbody fusion) patients (11).
One limitation of our study is its retrospective non-randomized nature. With roughly 14% of the total number of available patients included in the final analyses, this study experienced substantial loss to follow up. Loss to follow up was largely due to the absence of pre- and/or post-operative ODI scores. This limitation can introduce bias since it is not known if the observed outcomes are different from the unknown outcomes. Because the percentages of patients lost to follow up were the same for both cohorts, we assume that the results are the same as if there had been fewer lost patients.
Our primary outcomes in this study were functional status (ODI and ASA), costs, and complications. Future work should include fusion status and the relative rates of pseudoarthrosis and reoperation between the two cohorts.
Conclusions
By studying only degenerative spondylolisthesis, we were able to show no difference between PLF and PLF + TLIF in regards to functional outcome. Dural tears were more common in the PLF cohort, but other complications, reoperations, and blood loss were similar. Factors like OR time, implant cost and rhBMP-2 use considerably favor PLF over PLF + TLIF for degenerative spondylolisthesis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional approval for this study was obtained through Quorum Review IRB (approval ID: 29688).
References
- Eismont FJ, Norton RP, Hirsch BP. Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg 2014;22:203-13. [Crossref] [PubMed]
- Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 2016;374:1424-34. [Crossref] [PubMed]
- Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794-810. [Crossref] [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 2007;356:2257-70. [Crossref] [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295-304. [Crossref] [PubMed]
- Abdu WA, Lurie JD, Spratt KF, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976) 2009;34:2351-60. [Crossref] [PubMed]
- Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion. Z Orthop Ihre Grenzgeb 1982;120:343-7. [Crossref] [PubMed]
- Macki M, Bydon M, Weingart R, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg 2015;138:117-23. [Crossref] [PubMed]
- Jalalpour K, Neumann P, Johansson C, et al. A Randomized Controlled Trial Comparing Transforaminal Lumbar Interbody Fusion and Uninstrumented Posterolateral Fusion in the Degenerative Lumbar Spine. Global Spine J 2015;5:322-8. [Crossref] [PubMed]
- Al Barbarawi MM, Audat ZM, Allouh MZ. Analytical comparison study of the clinical and radiological outcome of spine fixation using posterolateral, posterior lumbar interbody and transforaminal lumbar interbody spinal fixation techniques to treat lumbar spine degenerative disc disease. Scoliosis 2015;10:17. [Crossref] [PubMed]
- Fujimori T, Le H, Schairer WW, et al. Does Transforaminal Lumbar Interbody Fusion Have Advantages over Posterolateral Lumbar Fusion for Degenerative Spondylolisthesis? Global Spine J 2015;5:102-9. [Crossref] [PubMed]
- Christensen A, Høy K, Bünger C, et al. Transforaminal lumbar interbody fusion vs. posterolateral instrumented fusion: cost-utility evaluation along side an RCT with a 2-year follow-up. Eur Spine J 2014;23:1137-43. [Crossref] [PubMed]
- Høy K, Bunger C, Niederman B, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J 2013;22:2022-9. [Crossref] [PubMed]
- Li FC, Chen QX, Chen WS, et al. Posterolateral lumbar fusion versus transforaminal lumbar interbody fusion for the treatment of degenerative lumbar scoliosis. J Clin Neurosci 2013;20:1241-5. [Crossref] [PubMed]
- Audat Z, Moutasem O, Yousef K, et al. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J 2012;53:183-7. [PubMed]
- Hu SS, Tribus CB, Diab M, et al. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am 2008;90:656-71. [PubMed]
- American Society of Anesthesiologists. ASA Physical Status Classification System. October 15, 2014. Available online: http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed September 12, 2017.
- Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 2008;8:968-74. [Crossref] [PubMed]
- Lauridsen HH, Hartvigsen J, Manniche C, et al. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord 2006;7:82. [Crossref] [PubMed]
- Carreon LY, Bratcher KR, Canan CE, et al. Differentiating minimum clinically important difference for primary and revision lumbar fusion surgeries. J Neurosurg Spine 2013;18:102-6. [Crossref] [PubMed]
- Clancy C, Quinn A, Wilson F. The aetiologies of failed back surgery syndrome: A systematic review. J Back Musculoskelet Rehabil 2017;30:395-402. [Crossref] [PubMed]
- Hartin NL, Mehbod AA, Joglekar SB, et al. Fusion risk score: evaluating baseline risk in thoracic and lumbar fusion surgery. Spine (Phila Pa 1976) 2013;38:E1616-23. [Crossref] [PubMed]
- Gottschalk MB, Premkumar A, Sweeney K, et al. Posterolateral Lumbar Arthrodesis With and Without Interbody Arthrodesis for L4-L5 Degenerative Spondylolisthesis: A Comparative Value Analysis. Spine (Phila Pa 1976) 2015;40:917-25. [Crossref] [PubMed]
- Chen NF, Smith ZA, Stiner E, et al. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine 2010;12:40-6. [Crossref] [PubMed]
- Desai A, Ball PA, Bekelis K, et al. Surgery for lumbar degenerative spondylolisthesis in Spine Patient Outcomes Research Trial: does incidental durotomy affect outcome? Spine (Phila Pa 1976) 2012;37:406-13. [Crossref] [PubMed]


