
Expandable spacers provide better functional outcomes than static spacers in minimally invasive transforaminal lumbar interbody fusion
Introduction
Transforaminal lumbar interbody fusion (TLIF) has been shown to be an effective surgical procedure for spondylolisthesis and related spinal disorders requiring surgical intervention (1-4). A critical component to the success of TLIF is the interbody spacer, which can help achieve sagittal correction. Since the preservation or restoration of sagittal alignment is a significant predictor in determining patient outcomes (5-7), increasing disc height, improving segmental lordosis, and achieving adequate indirect decompression are of the utmost importance.
Static interbody spacers have traditionally been used for spinal arthrodesis (8,9). Favorable outcomes have been reported following their use in anterior, posterior, and TLIF procedures (10-13). Most clinical outcome studies documented in the literature have focused on static interbody spacers, but expandable devices have recently become available.
Expandable spacers have been designed to be inserted at a minimized profile and expanded in situ for decreased trialing and iatrogenic endplate disruption secondary to impaction, in comparison to static devices. The potential surgical advantages of expandable devices include less neural retraction, decreased endplate damage, and less implant subsidence and/or migration. Functional clinical studies have provided evidence on the effectiveness of these new devices in improving patient outcomes compared to traditional devices. The objective of this study is to compare the clinical functional outcomes in patients treated with expandable spacers to those of static spacers in minimally invasive (MIS) TLIF.
Methods
Institutional Review Board approval was received and the patients were contacted by the principal investigator (PI) (ID: 1172515). Informed consent was given by all patients allowing the review of their data. A review was performed on the medical records of 99 patients who underwent TLIF, with 48 patients having been treated with a static and 51 having been treated with an expandable interbody spacer.
Outcome measures
Patient-reported clinical outcomes, visual analog scale (VAS) pain scores and Oswestry Disability Index (ODI), were collected from patient records, and subjects were contacted for final follow-up. Average final follow-up was 67.1±16.3 months for static patients and 43.0±4.2 months for expandable patients, for an overall average final follow-up of 54.7±16.9 months for all patients. All of the expandable interbody group patients completed patient reported outcomes through final follow-up, while 38 of 48 static interbody group patients completed patient reported outcomes through final follow-up. Radiographs were collected from the record system when available. Standard of care at the institution was for radiographs to be taken after 3 months only if the PI believed they were medically necessary, in order to reduce radiation exposure to patients. Therefore, radiographs were not collected for all patients at all time points.
Statistical analysis
Data were statistically analyzed using SPSS version 20.0.0 software for Windows (IBM Corp., Armonk, New York, USA). Frequency tables, descriptive statistics, and measures of central tendencies were calculated. Paired samples t-tests were used to compare outcomes over time, with P values of less than 0.05 considered significant. Independent sample t-tests were used to compare treatment groups, with the same significance threshold of 0.05.
Surgical technique
After general anesthesia, patients were positioned prone and secured on the operating table with adequate padding. A short 2–3-inch incision was made over the operative spinal level. Soft tissues were dissected down to bone in a MIS fashion. Tube retractors were placed, and facet joints and pars interarticularis were visualized. Percutaneous headless pedicle screws were placed bilaterally under C-arm fluoroscopy. This was followed by facetectomy under a microscope from an oblique approach (i.e., transforaminal), sometimes adding table rotation and angulating the tube medially to perform bilateral facetectomies if bilateral decompression was necessary. Next discectomy was performed and endplates were prepared. For patients receiving treatment with a static interbody spacer, repeated trials were used for sizing and preparation. For patients receiving treatment with expandable interbody spacers, trialing for implant height was not necessary as the height of the implant is adjusted in situ. The interbody spacer was filled with either cellular bone matrix or bone morphogenetic protein (BMP) and placement was completed via Kambin’s triangle. The rods were then passed bilaterally and set screws were secured. Pedicle screw and spacer placement was checked using fluoroscopy. Skin was washed and closed with sutures and skin glue in standard fashion. Patients were discharged either on the same day or 24 hours later.
Results
Indications for surgery included degenerative spondylolisthesis, spinal stenosis with or without recurrent herniated nucleus pulposus and degenerative disc disease. A breakdown of indications is displayed in Table 1. Of the total 99 patients, 48.5 percent were female, and there was no significant difference in gender proportions between study groups. There was no significant difference in age at surgery between the two study groups, with an average age of 61 years for all patients (Table 2). Patients treated with expandable interbody spacers had significantly less blood loss (81.7 vs. 36.2 mL, P<0.001) and shorter hospital stays (2.2 vs. 1.4 days) than patients treated with static interbody spacers. Operating room time for patients treated with expandable interbody spacers was 130.6 (±55.2) minutes, compared to 149.5 (±44.3) minutes for patients treated with static spacers. Despite a difference of 18.9 minutes on average, the difference did not reach significance (P=0.074).
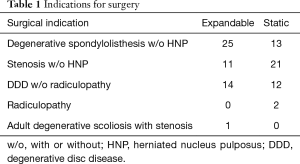
Full table

Full table
At 3-month follow-up, patients treated with expandable interbody spacers had significantly lower average ODI scores than patients with static interbody spacers (P<0.001, Table 3). At final follow-up, patients treated with expandable interbody spacers continued to show significantly lower average ODI scores than patients treated with static interbody spacers (P=0.016).
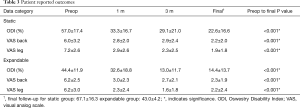
Full table
In the static cohort, there was one instance of deep vein thrombosis, one instance of vertigo, two instances of low blood pressure, one instance of wound infection, one instance of post-fall leg weakness, and one instance of hypoglycemia. In the expandable cohort, the only reported complication was an emergency room knee aspiration.
Disc height and neuroforaminal height increased significantly (P<0.05) from baseline at the 3-month follow-up time point for both interbody spacers. The expandable group had significantly greater neuroforaminal height (22.3 vs. 20.1 mm) than the static group (P<0.05).
The standard of care for patients at the study site did not require radiographs to be taken past 3-month postoperative follow-up unless the patient reported a recurrence of severe low back pain or other symptoms. Only 3 of 51 (6%) patients from the expandable group had to have imaging taken at >24 months, compared to 12 of 48 (25%) patients in the static group, which was significant (P<0.05). The three patients with a recurrence of pain in the expandable group each returned within 4 years postoperative. Ten of the 12 patients with a recurrence of pain in the static group returned within 4 years postoperative. Furthermore, 4 of the 12 static patients required additional surgery, while the three expandable group patients received only conservative treatment.
Discussion
This study illustrates favorable radiographic and functional outcomes after MIS TLIF using either expandable or static interbody spacers. Most TLIF-oriented studies focus on radiographic outcomes. However, patient-reported outcome measures are recognized (such as ODI and VAS pain scores) as the most appropriate instruments to assess the effectiveness of healthcare interventions from the patient’s perspective, and are sometimes used as metrics for healthcare delivery performance (14,15).
A small number of recent studies have evaluated disc and neuroforaminal heights in patients with expandable interbody devices. Kim et al. (16) assessed the radiographic outcomes in 50 patients and reported that MIS TLIF with an expandable interbody device led to a long-lasting increase in disc height, but only a transient increase in foraminal height. Similar to Kim et al., the current study demonstrated that MIS TLIF with an expandable interbody device increased disc height and neuroforaminal heights by 48.7% on average. MIS TLIF with static interbody devices led to a 36.8% smaller increase in disc height. Regardless of radiographic parameters, a key finding of this study was the improvement of functional outcomes. VAS back scores decreased significantly by 64.5%, and ODI scores decreased significantly by 67.5% in the expandable group compared to the static group, with a decrease of 63.3% and 60.4% in VAS and ODI scores, respectively.
Another key finding of the study is the rate of follow-up between both groups. The standard of care for this site was to minimize radiation exposure by ordering X-rays only if clinically indicated. The most common chief complaint was recurring back and leg pain. Only 6% of patients in the expandable group had recurrence of symptoms in contrast to 25% of patients in the static group at final follow-up. Despite the difference in average follow-up (67.1±16.3 months for static group patients and 43.0±4.2 months for expandable group patients), the eight static group patients and three expandable group patients returned within 4 years postoperative.
Similar results in functional outcomes were reported in one other study. Hawasli et al. (17) compared radiographic and clinical outcomes between expandable and static interbody spacers. A total of 48 MIS TLIFs were performed. The expandable group had a greater and more sustained increase in disc height when compared to the static group. Foraminal height increased with the expandable group but not with the static group. In the expandable group, ODI improved more significantly compared to the static group. Both disc height and segmental lordosis were correlated with improved clinical outcomes in the study (17). The results of Hawasli et al. are comparable to the results reported here, and support the effectiveness of expandable interbody spacers generally.
Limitations of this study include a small sample size and limited follow-up X-rays, because they were ordered only if clinically indicated in order to reduce radiation exposure in accordance with the standard of care for this site.
Conclusions
This study adds to current body of research demonstrating that expandable interbody spacers provide comparable height restoration and clinical outcome scores when compared to static interbody spacers.
Acknowledgments
None.
Footnote
Conflicts of Interest: MA Kremer and J Alferink are consultants of Globus Medical Inc. T Shirk and C Ledonio are employees of Globus Medical Inc. S Wynsma has no conflicts of interest to declare.
Ethical Statement: Institutional Review Board approval was received and the patients were contacted by the Principal Investigator (PI) (ID: 1172515). Informed consent was given by all patients allowing the review of their data. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ghasemi AA. Transforaminal lumbar interbody fusion versus instrumented posterolateral fusion In degenerative spondylolisthesis: An attempt to evaluate the superiority of one method over the other. Clin Neurol Neurosurg 2016;150:1-5. [Crossref] [PubMed]
- de Kunder SL, van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J 2017;17:1712-21. [Crossref] [PubMed]
- Glassman SD, Carreon LY, Ghogawala Z, et al. Benefit of Transforaminal Lumbar Interbody Fusion vs Posterolateral Spinal Fusion in Lumbar Spine Disorders: A Propensity-Matched Analysis From the National Neurosurgical Quality and Outcomes Database Registry. Neurosurgery 2016;79:397-405. [Crossref] [PubMed]
- Lee N, Kim KN, Yi S, et al. Comparison of Outcomes of Anterior, Posterior, and Transforaminal Lumbar Interbody Fusion Surgery at a Single Lumbar Level with Degenerative Spinal Disease. World Neurosurg 2017;101:216-26. [Crossref] [PubMed]
- Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024-9. [Crossref] [PubMed]
- Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30:682-8. [Crossref] [PubMed]
- Rodgers JA, Gerber EJ, Lehmen JA, et al. Clinical and Radiographic Outcome in Less Invasive Lumbar Fusion: XLIF at Two Year Follow-Up. J Spine Neurosurg 2013. [Crossref]
- Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics 1988;11:931-4. [PubMed]
- McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am 1999;81:859-80. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989.
- Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 2013;38:1853-61. [Crossref] [PubMed]
- Sharma AK, Kepler CK, Girardi FP, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech 2011;24:242-50. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976) 2010;35:S302-11. [Crossref] [PubMed]
- Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018;9:353-67. [Crossref] [PubMed]
- Weldring T, Smith SM. Patient-Reported Outcomes (Pros) and Patient-Reported Outcome Measures (Proms). Health Serv Insights 2013;6:61-8. [Crossref] [PubMed]
- Kim CW, Doerr TM, Luna IY, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion Using Expandable Technology: A Clinical and Radiographic Analysis of 50 Patients. World Neurosurg 2016;90:228-35. [Crossref] [PubMed]
- Hawasli AH, Khalifeh JM, Chatrath A, et al. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus 2017;43:E10. [Crossref] [PubMed]


