Microendoscope-assisted posterior lumbar interbody fusion: a technical note
Introduction
Surgical interbody fusion is an effective treatment option to stabilize the motion of the painful segments and to provide decompression of the neural elements. There are several surgical options available for lumbar interbody fusion, and each technique has its own inherent advantages and disadvantages (1).
Traditional open surgery techniques for posterior lumbar fusion are widely accepted methods, but many authors have documented adverse effects of the extensive tissue damages. In 2003, Foley et al. (2) described minimally invasive transforaminal lumbar interbody fusion (MI-TLIF), subsequently many publications have reported the advantages of such an approach, with good outcomes (3-7).
Since 2008 we have performed microendoscope-assisted posterior lumbar interbody fusion (ME-PLIF) using an 18-mm tubular retractor. The technique includes all steps (decompression, curettage of the endplate, bone grafting, and insertion of the cage), other than pedicle screw (PS) insertion, uses only a microendoscopic system, and has not been reported on so far. The purpose of this study is to document the new technique and the clinical and radiological outcomes.
Methods
Between January 2011 and December 2011, a total of 48 patients underwent one level ME-PLIF performed by a surgeon (H Inanami) in our hospital, and we followed up 46 patients (95.8%). All of them underwent ME-PLIF using the tubular retractor system (METRx, Medtronic Sofamor Danek, Memphis, TN, USA). The minimum follow-up period was 25 (range, 25–39) months. All patients had a pre-operative evaluation with plain lumbar spine radiography, magnetic resonance (MR) imaging, and a computed tomography (CT) scan. The indications for surgery were spondylolisthesis, spondylolysis, degenerated disc disease (DDD), intra-extra foraminal stenosis, and lumbar disc herniation. Both during the perioperative period and post-surgery, the patients were monitored for complications and were followed regularly by the surgeon (e.g., for intraoperative faults, cage migrations, new or worsening neurological deficits, wound infections, etc.). For clinical outcomes, the Oswestry Disability Index (ODI) and the Japanese Orthopedic Association (JOA) scores were used to assess the pre-operative and post-operative (2 years post-surgery) pain and disability status of the patients. For radiological outcomes, the fusion rates based on the Bridwell fusion grading system (5,8) were evaluated using plain radiography or a CT scan at the most recent follow-up point. All data were collected prospectively, and this study is a retrospective review of the data.
Surgical technique
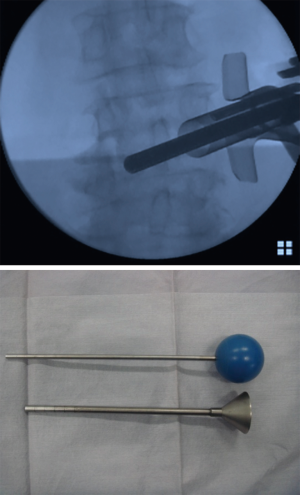
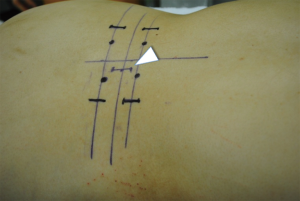
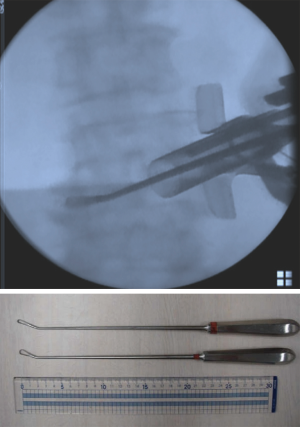
First, we start with the decompression. The level of the intervertebral disc was marked, and a 20-mm incision was made into the skin 15 mm outside from the midline on the symptomatic side (Figure 1). Using fluoroscopic guidance, the tubular retractor (18 mm) was placed on the lamina-facet junction overlying the disc space, and the operation was performed with the microendoscope connected to the tubular retractor. Subsequently all the inferior articular process and part of the superior vertebra were resected using a chisel until the flavum was removed. When the superior articular process appears, part of the superior articular process and part of the inferior vertebra were removed using a chisel and Kerrison rongeur until the root was decompressed sufficiently. This process allows for direct neural decompression, and the nerve root could be gently retracted medially. After the decompression was completed, the intervertebral disc was thoroughly removed using an angled curette, a ring curette to the opposite side (Figure 2). The autologous bone was crushed and was then inserted using the bone funnel (Figure 3). If the autologous bone was inadequate, a granular artificial bone was also used. Subsequently, a boomerang type cage was inserted using fluoroscopic guidance. Following ending of the microendoscopic operation, screws were inserted percutaneously using fluoroscopic guidance (Figure 4). Compression is applied to this construct before final tightening, restoring lordosis and providing compression of the bone graft in the middle column.
Results
The mean age of the patients was 61.4 (range, 36.0–86.0) years, and the ratio of men to women was 27 to 19. The group consisted of 2 patients who were operated on at L34, 29 at L45, and 15 at L5S1. The mean operation time was 102 (range, 59–162) min, and the mean blood loss was 86 (range, small amounts–315) mL (Table 1).
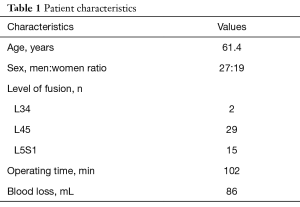
Full table
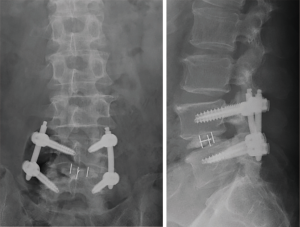
The clinical data collected prior to and post-ME-PLIF are presented in Table 2. The average pre- and post-operative ODI scores were 22.1 and 9.7, respectively, with an improvement rate of 56.1%. The pre- and post-operative JOA scores were 9.8 and 16.4, respectively, with an improvement rate of 50%. Regarding the radiological outcomes, 31 cases had a grade 1 fusion, and 14 cases had a grade 2 fusion based on the Bridwell fusion grading system. There was one grade 3 case, which required revision surgery owing to infection. There were no grade 4 cases (Table 3). We identified one case in which the vertebral body moved 3 mm or more on functional imaging, and one in which the transparence around the PS occurred as pseudarthrosis, though there were no cases of pseudarthrosis.

Full table
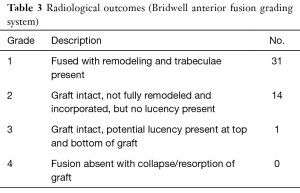
Full table
Regarding complications, 1 case (2.2%) developed a deep wound infection, which became obvious 2 weeks post-surgery and revision surgery was performed to remove all implants. Four (8.7%) cases had a dural tear and required dural suturing using the aid of a microscope, though they experienced no severe nerve damage. There was no incident of cage migration (Table 4).

Full table
Discussion
Our new procedure, ME-PLIF, which was performed from decompression to the insertion of the cage using a microendoscopic system, was considered to be a beneficial method. While MI-TLIF is under direct view or uses a microscope, ME-PLIF is performed under a microendoscope. The use of the microendoscope makes it possible to obtain smaller skin tears and a good field of view. In addition, it is possible to change the tubular retractor to various angles (this is difficult under direct viewing or under a microscope), and the operation on the opposite side becomes easy. On the other hand, so far, many reports on MI-TLIF have been produced since Foley et al. first described the technique in 2003 (2), and stable results have been shown using this method. Basically, sequential dilators are used, and the distal end of a 22- or 26-mm diameter tube of appropriate length is positioned over the facet joint complex. Interbody is inserted into the disc space via the METRx tube. The tubular retractor is removed, and a PS-rod construct is inserted. Compared to open surgery, reduced bleeding, a shorter operation time, and greater improvements in post-operative outcomes have been reported with MI-TILF (3-7). On the other hand, the microendoscopic discectomy (MED) has been used as a minimally invasive surgical method to treat lumbar herniated discs since 1997, when it was first introduced by Foley and Smith (9). Good outcomes have been reported using this method (10,11). We hypothesized that combining these two methods would result in a less invasive procedure.
We performed all steps, except PS insertion, using only a microendoscopic system called the METRx (Medtronic Sofamor Danek, Memphis, TN, USA). The use of an 18-mm tube makes it possible to make small incisions. In addition, the operation time and blood loss are minimal compared to the past reports of MI-TLIF by Park et al. (3) and Peng et al. (5). The microendoscope has a better visual field and is easier for the surgeons to handle compared to the microscope. But the two-dimensional view and hand-eye spatial separation of the microendoscopic view can also be extremely disorienting (10). Ensuring satisfactory technique will obviously require additional training and experience. The patients showed more improvements in their ODI and JOA scores post-surgery compared with their pre-operative scores. In our case, no case of pseudarthrosis or cage migration was found. This suggests that this procedure may enable thorough curettage of the cartilage endplate and bone grafting. Park et al. reported that solid fusion could be achieved using minimally invasive techniques in the same way as the traditional open surgery approach (3). The rate of dural
Conclusions
The short-term use of our novel technique, ME-PLIF using only a microendoscopic system, provides satisfactory results. Although knowledge about long-term outcomes is needed, this appears to be both a safe and minimally invasive option for the treatment of lumbar spine degeneration.
Acknowledgements
We would like to thank all the operating room staff for their technical assistance, and the medical records clerks who helped collect patient data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by ethics committee of the Iwai Medical Foundation, and informed consent was obtained from the patients for publication of this study and any accompanying images.
References
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28:S26-35. [Crossref] [PubMed]
- Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976) 2007;32:537-43. [Crossref] [PubMed]
- Lee KH, Yue WM, Yeo W, et al. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J 2012;21:2265-70. [Crossref] [PubMed]
- Peng CW, Yue WM, Poh SY, et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2009;34:1385-9. [Crossref] [PubMed]
- Shunwu F, Xing Z, Fengdong Z, et al. Minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases. Spine (Phila Pa 1976) 2010;35:1615-20. [Crossref] [PubMed]
- Fan G, Zhang H, Guan X, et al. Patient-reported and radiographic outcomes of minimally invasive transforaminal lumbar interbody fusion for degenerative spondylolisthesis with or without reduction: A comparative study. J Clin Neurosci 2016;33:111-8. [Crossref] [PubMed]
- Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410-8. [Crossref] [PubMed]
- Foley KT, Smith MM. Microendoscopic discectomy. Tech Neurosurg 1997;3:301-7.
- Wu X, Zhuang S, Mao Z, et al. Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine (Phila Pa 1976) 2006;31:2689-94. [Crossref] [PubMed]
- Schick U, Döhnert J, Richter A, et al. Microendoscopic lumbar discectomy versus open surgery: an intraoperative EMG study. Eur Spine J 2002;11:20-6. [Crossref] [PubMed]
- Parker SL, Adogwa O, Witham TF, et al. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg 2011;54:33-7. [Crossref] [PubMed]