
Ultrasound diagnosis and therapeutic intervention in the spine
Introduction
Spinal pathology affects many people in the United States, as approximately 25% of the adult population suffers from low back pain and an additional 15% experiences neck pain (1,2). This accounts for 2% of all physician visits and amounts to annual healthcare costs of $85.9 billion (1). The human spine is composed of multiple different structural elements with varying attributes of thickness, depth, rigidity, and location, together contributing to spinal function (3). To adequately assess these elements, use of imaging studies for diagnosis and management of spinal pathology continues to grow rapidly (4-8). The four predominant imaging modalities are CT, MRI, plain radiographs, and ultrasound9. CT, MRI, and radiographs offer benefits in terms of resolution, widespread use in practice, and consistency in image acquisition and interpretation (1,9). However, they do not offer several advantages compared to ultrasound such as portability, affordability, rapidity, and ability to easily obtain dynamic images (9). Ultrasound has less variability than MRI and CT in terms of accessibility of the equipment (10,11), and has demonstrated efficacy in imaging the musculature, bones, intervertebral discs, nerve roots, the spinal cord, and spinal curvature and mobility (9,12,13). In some areas, ultrasound has shown comparable results to gold standard modalities like MRI, suggesting possible utility in field situations where MRI is not available (9). These features make ultrasound suitable for use in situations such as emergency trauma assessment, image guided therapeutic intervention in non-surgical settings, utilization in underserved areas with limited healthcare capital, and in remote or otherwise resource-limited environments such as wilderness field stations, combat settings, and aerospace medicine (9,13-15). Spinal pathology is a key topic in aerospace medicine for conditions including chronic low back pain, intervertebral disc decompression, spinal curvature changes, and neck fatigue, and ultrasound may aid in diagnosis and management of these conditions (16-18). This goal of this review is to investigate the efficacy of ultrasound for diagnostic imaging and therapeutic intervention in spinal pathology.
Methods
A systematic review was conducted on currently available information and published literature of human and animal studies regarding ultrasound imaging of the spine, using recommendations and PRISMA guidelines (19). The search query used was (“cervical” or “thoracic” or “thoracolumbar” or “lumbar”) and (“spine” or “spinal”) and (“ultrasound” or “ultrasonography”). Databases included in the literature search were PubMed and MEDLINE. All titles obtained from these search criteria were reviewed. Studies published in a language other than English without available translation were discarded. Articles regarding the topic of interest that did not address ultrasound as a modality to image the spine were discarded. The remainder were reviewed in their entirety. The references of these manuscripts were also searched to identify additional applicable studies. Google Scholar was used as a secondary resource to locate supplementary papers relevant to the topic of interest.
Results
Using these methods, 3,630 references were identified that met search criteria and addressed the topics of interest. In total, 1,485 of these had the search terms present in the title or abstract. Of these, 170 studies were published in a non-English language without readily available translation and were discarded. An additional 196 studies involving non-human subjects were discarded. Thirteen references were discarded for abstract unavailability. A total of 550 articles greater than 20 years old were discarded. And 548 of the remaining articles were identified to address topics out of the scope, such as fetal ultrasound, cadaveric imaging, etc. and were discarded. The remaining 73 articles were reviewed in their entirety. The references of these manuscripts were also searched to identify additional applicable studies. Literature obtained includes in vivo studies, case studies, technical reports, white papers, device operating manuals, and review articles (Figure 1).
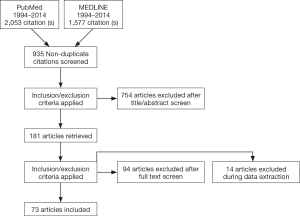
Twenty-one supplementary papers relevant to the topic from Google Scholar were added to the set and included in review.
The following subsections discuss ultrasound efficacy in diagnosis and therapeutic intervention for the different elements of the spine.
Efficacy of imaging in diagnosis
Musculature
Muscular anatomy imaging via ultrasound was studied in six of the reviewed articles. Muscular visualization is clinically relevant for activity related neck stress, spinal pathology compensation, and chronic neck pain (17,20,21). Leung et al. (22) showed that the posterior neck muscles and their internal architecture in the cervical spine are easily and effectively imaged. Clear pictures can be gathered of these structures using common modern ultrasound equipment (22). Specific attributes of the musculature such as cross-sectional area, thickness, width, and muscle depth can be visualized in the posterior paraspinal musculature (rectus capitis posterior major, oblique capitis superior, semispinalis capitis, multifidus, erector spinae, and splenius capitis) at rest and during contraction; these measurements were found to be reliable when confirmed against other imaging modalities (21,23-27). Lee et al. compared ultrasound measurements of muscle thickness at rest to corresponding MRI measurements and found similar measurement reliability and better visibility with ultrasound (at C4, C5, and C6 levels: ultrasound =0.67±0.14, 0.70±0.20 and 0.73±0.09 cm, respectively; MRI =0.70±0.12, 0.67±0.15 and 0.70±0.06 cm, respectively) (28). The skin overlying the posterior musculature can also reliably be assessed for thickness (29).
Spinal curvature
Anterior-posterior and lateral curvature measurement of the spine has significant clinical implications for many physicians, including orthopedic surgeons, neurologists, neurosurgeons, and emergency medicine physicians (6,30-33). Neurologic and musculoskeletal pathology resulting from spinal deformity can be treated more effectively with appropriate imaging (31,34,35). The Cobb angle as measured on radiographs is the gold standard for obtaining degrees of curvature, but ultrasound imaging can also effectively be used (6). Prushansky et al. found that this modality gives results that are highly reproducible in young healthy adults with a precision of measurement of 1.2˚, 2.6˚, 3.8˚ for the thoracic, lumbar, and cervical regions, respectively (36). A study by Gercek et al. assessing cervical flexion/extension during endotracheal intubation demonstrated precision with their own measurement of 2˚ (37). Chleboun et al. reported excellent reliability of ultrasound for flexion/extension measurement in the lumbar spine; correlation to MRI was 0.94 (38). Reliability of curvature measurement via ultrasound has also been demonstrated by proxy in other clinical settings. Haque et al. outlined its use in evaluating torticollis in pediatric patients (39). Teng et al. used it to confirm positioning after manual head manipulation by physical therapists (40).
Range of motion
Range of motion testing is employed clinically to assess for spinal pain and possible damage, often without supplementary imaging. However, imaging can shed further diagnostic light, especially if mechanical problems are suspected (37,41). Ultrasound utilized dynamically can investigate the shape of the spine, its motion patterns and relative mobility, and is particularly effective in assessing movement characteristics of the cervical spine (42). As range of motion varies based on body position, Strimpakos et al. (41) showed that inter- and intra-examiner reliability in assessing 3D range of motion is more effective with the subject standing rather than sitting (correlation >0.86 and >0.79, respectively). Further study also demonstrated that range of motion assessment via ultrasound is reliable when compared to plain radiographs as a source of validation (41).
Bones and discs
The bones and intervertebral discs of the spine are affected in a variety of settings including chronic deterioration with aging, traumatic injury, and neurologic spinal pathology (12,32,43,44). Reliable imaging of bone can inform a diagnosis of injury, fracture, or degenerative disease, and may further be employed as a marker for changes in disc height, compression, or extrusion (12,32,43). Marshburn et al. demonstrated how ultrasound used aboard the International Space Station can image cervical vertebrae and intervertebral discs in the C7–T1 region without difficulty or obstruction from vascular structures (9). Ledsome et al. revealed that ultrasound can reliably measure the intervertebral disc distance (45). Finlayson et al. demonstrated that the transverse processes can be clearly identified in the coronal plane (27,45-47). Spinous processes were assessed with greater identification by ultrasound as compared to palpation (48-50). In a study by Ungi et al. (51) articular processes and pedicles were identified and pedicle screw planned placement was tracked by ultrasound with comparable accuracy to CT guided pedicle screw planned tracks. In assessing spinal trauma, Mueller et al. (44) found ultrasound reliable in thoracolumbar burst fracture repositioning when compared to CT. In a prospective study by Vordemvenne et al. (35), ultrasound was also found to be effective in detecting traumatic lesions to the posterior ligamentous complex with 99% sensitivity and 75% specificity (P<0.05), which was comparable to MRI.
Canal structures
Based on the review parameters, evidence was not found specifically assessing the quality of ultrasound imaging of spinal canal pathology in vivo. Multiple studies demonstrating efficacy of ultrasound in this regard used cadaveric models. Although efficacy of diagnosing pathology has not been validated in the articles reviewed, ultrasound was shown to be effective in visualizing attributes of the canal including vertebral level, canal, corpus vertebrae, subarachnoid space, ligamentum flavum, and dura mater (48,51-59).
Ultrasound has been used to accurately assess the size of the canal for determination of possible stenosis or cord compression (44). Edelbauer et al. highlighted that ultrasound can image sensitively into the canal to identify dura mater and detect subdural hematomas (60). Ultrasound has also been used to identify changes to the spinal cord itself, as found by Provoost et al. in a study where they detected a syrinx in the spinal medulla (33).
Use of ultrasound to aid therapeutic intervention
Injections
Numerous clinical procedures involve introduction of instruments, needles, or devices into or around the spine, and ultrasound has been used to enhance physician identification of structures (61). Kim et al. (62) showed that ultrasound is effectively used in cervical epidural steroid injections during these procedures. Ultrasound illustrated high imaging efficacy in transverse and longitudinal views of the C6/C7 area to measure the skin to dura depth, with correlations of 0.9272 and 0.9268 respectively to actual needle depth (52,62-64). Ozer et al. posited that precision of measuring this depth is high enough to derive a statistical model (accounting for BSA, height, weight, and age) for extrapolating values for the L4–L5 interspace (65).
Ultrasound usage in performing nerve blocks was discussed in multiple studies. Bozeaart et al. demonstrated efficacy of nervous structure identification as they visualized spinal exiting nerve roots (66,67). Herring et al. identified branches of spinal roots in visualizing the ventral rami of C1–C4 lateral to the transverse processes, forming the cervical plexus (68). Ilfeld et al. (69) showed similar nerve root visualization with ultrasound in the lumbar spine. Chin et al. (70) showed that ultrasound reduced the technical difficulty and improved clinical efficacy, but cautioned that further validation is still needed (71). In addition to visualizing the nerves themselves, ultrasound use allowed clear identification of the foramen, adjacent structures, interlaminar spaces, epidural/intrathecal spaces, and pedicles (72-75). Facet and para-radicular injections were also aided by administration under ultrasound imaging guidance (47,76-78). Ultrasound user performance was assessed by Finlayson et al., showing reliability for C5–C6 medial branch blocks with a user performance time of 248.8±92.7 seconds for identification of the C7 transverse process and injection of block (46).
Ultrasound-aided injection for management of spinal pathology has been compared to other imaging modalities. Jee et al. found ultrasound to be as effective as fluoroscopy when employed for transforaminal injections (73). Galiano et al. determined that ultrasound was 90% accurate in identification of facet joints when compared against CT imaging (79,80). Obernauer et al. in a similar study assessed speed of injection administration and found that ultrasound was superior to CT guidance for facet joint injections with quicker time to final needle placement (04:46 versus 11:12 and 05:49 versus 14:32 for 1 and 2 levels, respectively) (81).
Lumbar puncture
Lumbar puncture is used to augment clinical suspicion for conditions like meningitis and subarachnoid hemorrhage (82). Duneic et al. (83) showed that lumbar puncture accuracy is improved with ultrasound compared to manual palpation. One study by Peterson et al. contradicts this, asserting that there is no advantage to ultrasound localization for routine lumbar puncture (84). Identification of the intervertebral spaces is typically done through manual palpation, but variability in body habitus can obfuscate structure identification in up to 30% of cases (48,85,86). Ultrasound addresses this challenge by providing an accessible, portable, and affordable visual aid (70,87).
Epidural anesthesia
Epidural anesthesia is commonly used in child delivery and surgical procedures, and requires introduction of a catheter into the spine (67,88). According to the CDC National Vitals and Statistics Report on Epidural and Spinal Anesthesia Use During Labor, approximately 60% of mothers with vaginal deliveries used epidural anesthesia (89). This large quantity of patients may benefit from the reliable visualization offered by ultrasound compared to landmark identification (34,90,91). Epidural catheter placement can be aided by ultrasound, resulting in improved success rate for visualizing the dura and epidural space (49,57,59,74,85,88,92-94). Chin et al. found that ultrasound-aided imaging improves the success rate of spinal anesthesia in adults by 100% (70). Studies have evaluated differing ultrasound techniques, devices, and beams to determine if differences in visual clarity of anatomical features exist, but no significant differences were reported (95-98).
Discussion
Ultrasound has been cited in the literature extensively for utilization as a clinical aid, but not in regard to rigorous analysis of its effectiveness. In the body of research reviewed, nearly all of the structures within the spine have been shown to be clearly visible via ultrasound imaging including musculature, bones and intervertebral discs, nerve roots, the spinal cord, dura mater, facet joints, and foramen (9,12,13). Functional aspects of the spine can also be imaged with ultrasound, with reliability demonstrated in spinal curvature and mobility assessment (38,42). Ultrasound has been used extensively and with accuracy in therapeutic spinal injections (68,81). These studies demonstrate that ultrasound effectively provides visual assessment of the structure and function of the spine and is useful in assisting clinical diagnosis and therapeutic intervention.
Regarding bony work, the preliminary study by Ungi et al. (51) showed comparable accuracy of ultrasound to CT guidance for planning pedicle screw tracks. This is an interesting area for further investigation; if ultrasound can show similar consistent reliability in accurate hardware placement, it may become a very appealing intra-operative modality considering the lack of damaging radiation exposure to both patient and provider. Also intriguing are the studies by Mueller et al. (44) and Vordemvenne et al. (35) assessing ultrasound usage in thoracolumbar burst fracture repositioning and evaluating posterior ligamentous injuries. Despite promising results, it is hard to imagine ultrasound ubiquitously replacing modalities such as CT and MRI in general trauma evaluation. However, employing ultrasound in developing countries or more remote resource stricken settings that simply do not have access to advanced cross-sectional imaging may be a reasonable development. For instance, ultrasound technology has already been used aboard the International Space Station (9), which is arguably one of the most remote and difficult to access areas in terms of resources, with transportation costs of over 10,000 USD per kilogram (99) to the station.
As mentioned above, spinal curvature, mobility, and range of motion have been accurately assessed with ultrasound (38,42). Yet further characterization of these parameters in both normal and pathologic states with ultrasound is needed prior to general adoption in assessing for stable versus unstable injuries after trauma. If this can be accomplished, ultrasound may serve as a valuable and affordable initial survey to identify those patients requiring evacuation to higher level trauma centers.
A limitation to broader ultrasound utilization is the uncertainty in teaching this imaging technique. The literature does not form a strong conclusion whether it can be taught effectively or not. For instance, Margarido et al. showed that 20 supervised trials plus teaching sessions were not enough for the participants to achieve competence in different aspects of ultrasound assessment of the lumbar spine (100). Conversely, Deacon et al. showed after a standardized educational intervention, anesthetic trainees are able to identify a lumbar interlaminar space easily and can measure the depth to the posterior complex after a reasonable number of additional practice scans (101). More robust educational programs (79) and broader availability of ultrasound equipment in training institutions may yield increased fluency in the clinical usage and interpretation of ultrasound acquired images.
Despite the literature presented in the current study, ultrasound is overall cited infrequently for diagnosis of spinal pathology. Less than 10% of the articles reviewed dealt with topics of ultrasound as a spinal diagnostic modality. In modern usage, ultrasound imaging is still most often employed as an aid for procedures involving injection or introduction of needles about the spine. This widespread utilization in procedural guidance demonstrates the ease with which spinal structures are visualized with ultrasound. Once adequate visualization can be established consistently with an imaging modality, diagnostic ability follows. In considering several promising studies delineated above and the portability, affordability, and dynamic imaging characteristics of ultrasound, the authors have the following recommendations: (I) introduce standardized and reproducible educational programs for ultrasound performance and interpretation; (II) continued utilization to aid therapeutic interventions about the spine given the enhanced landmark identification and accuracy; (III) additional studies comparing diagnostic ability of ultrasound against CT and MRI, particularly for (i) vertebral compression fractures and intervertebral disc pathology by assessing vertebral height differences; and (ii) assessing posterior ligamentous complex stability, as this is a common decision point between operative spinal stabilization versus non-operative management (102); (IV) further investigation of ultrasound to guide intra-operative hardware placement, as initial promise has been shown; (V) despite some encouraging results, take caution against premature broad ultrasound implementation for spinal diagnostics due to the lack of a large body of consistent evidence in different settings of spine pathology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Flynn TW, Smith B, Chou R. Appropriate Use of Diagnostic Imaging in Low Back Pain - A Reminder That Unnecessary Imaging May Do as Much Harm as Good. J Orthop Sports Phys Ther 2011;41:838-46. [Crossref] [PubMed]
- American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States. Second Edi. Rosemont, IL: 2008.
- Rauschning W. Surgical Anatomy of the Spine Revisited. Spine 2017;42:S1-S2. [Crossref] [PubMed]
- Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976) 2008;33:E990-3. [Crossref] [PubMed]
- Bono CM, Vaccaro AR, Fehlings M, et al. Measurement techniques for upper cervical spine injuries: consensus statement of the Spine Trauma Study Group. Spine (Phila Pa 1976) 2007;32:593-600. [Crossref] [PubMed]
- Bono CM, Vaccaro AR, Fehlings M, et al. Measurement techniques for lower cervical spine injuries: consensus statement of the Spine Trauma Study Group. Spine (Phila Pa 1976) 2006;31:603-9. [Crossref] [PubMed]
- Bono CM, Schoenfeld A, Gupta G, et al. Reliability and reproducibility of subaxial cervical injury description system: a standardized nomenclature schema. Spine (Phila Pa 1976) 2011;36:E1140-4. [Crossref] [PubMed]
- Chou R, Deyo RA, Jarvik JG. Appropriate Use of Lumbar Imaging for Evaluation of Low Back Pain. Radiol Clin North Am 2012;50:569-85. [Crossref] [PubMed]
- Marshburn TH, Hadfield CA, Sargsyan AE, et al. New heights in ultrasound: first report of spinal ultrasound from the international space station. J Emerg Med 2014;46:61-70. [Crossref] [PubMed]
- Lysdahl KB, Børretzen I. Geographical variation in radiological services: a nationwide survey. BMC Health Serv Res 2007;7:21. [Crossref] [PubMed]
- Bono CM, Schoenfeld A, Rampersaud R, et al. Reproducibility of radiographic measurements for subaxial cervical spine trauma. Spine (Phila Pa 1976) 2011;36:1374-9. [Crossref] [PubMed]
- Johnston SL, Campbell MR, Scheuring R, et al. Risk of Herniated Nucleus Pulposus Among U.S. Astronauts. Aviat Space Environ Med 2010;81:566-74. [Crossref] [PubMed]
- Sargsyan AE, Hamilton DR, Jones JA, et al. FAST at MACH 20: clinical ultrasound aboard the International Space Station. J Trauma 2005;58:35-9. [Crossref] [PubMed]
- Kerstman EL, Scheuring RA, Barnes MG, et al. Space Adaptation Back Pain: A Retrospective Study. Aviat Space Environ Med 2012;83:2-7. [Crossref] [PubMed]
- Scheuring RA, Mathers CH, Jones JA, et al. Musculoskeletal Injuries and Minor Trauma in Space: Incidence and Injury Mechanisms in U.S. Astronauts. Aviat Space Environ Med 2009;80:117-24. [Crossref] [PubMed]
- Kershner D, Binhammer R. Intrathecal ligaments and nerve root tension: possible sources of lumbar pain during spaceflight. Aviat Space Environ Med 2004;75:354-8. [PubMed]
- Harrison MF, Neary JP, Albert WJ, et al. Neck Pain and Muscle Function in a Population of CH-146 Helicopter Aircrew. Aviat Space Environ Med 2011;82:1125-30. [Crossref] [PubMed]
- Sayson JV, Hargens AR. Pathophysiology of low back pain during exposure to microgravity. Aviat Space Environ Med 2008;79:365-73. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6. [Crossref] [PubMed]
- Jesus-Moraleida FR, Ferreira PH, Pereira LS, et al. Ultrasonographic analysis of the neck flexor muscles in patients with chronic neck pain and changes after cervical spine mobilization. J Manipulative Physiol Ther 2011;34:514-24. [Crossref] [PubMed]
- Rezasoltani A, Ahmadipoor A, Khademi-Kalantari K, et al. The sign of unilateral neck semispinalis capitis muscle atrophy in patients with chronic non-specific neck pain. J Back Musculoskelet Rehabil 2012;25:67-72. [Crossref] [PubMed]
- Leung YL, Roshier AL, Johnson S, et al. Demonstration of the appearance of the paraspinal musculoligamentous structures of the cervical spine using ultrasound. Clin Anat 2005;18:96-103. [Crossref] [PubMed]
- Stokes M, Hides J, Elliott J, et al. Rehabilitative ultrasound imaging of the posterior paraspinal muscles. J Orthop Sports Phys Ther 2007;37:581-95. [Crossref] [PubMed]
- Lin YJ, Chai HM, Wang SF. Reliability of thickness measurements of the dorsal muscles of the upper cervical spine: an ultrasonographic study. J Orthop Sports Phys Ther 2009;39:850-7. [Crossref] [PubMed]
- Rezasoltani A, Nasiri R, Faizei AM, et al. The variation of the strength of neck extensor muscles and semispinalis capitis muscle size with head and neck position. J Bodyw Mov Ther 2013;17:200-3. [Crossref] [PubMed]
- Brenner AK, Gill NW, Buscema CJ, et al. Improved activation of lumbar multifidus following spinal manipulation: a case report applying rehabilitative ultrasound imaging. J Orthop Sports Phys Ther 2007;37:613-9. [Crossref] [PubMed]
- Darrieutort-Laffite C, Hamel O, Glémarec J, et al. Ultrasonography of the lumbar spine: sonoanatomy and practical applications. Joint Bone Spine 2014;81:130-6. [Crossref] [PubMed]
- Lee JP, Tseng WY, Shau YW, et al. Measurement of segmental cervical multifidus contraction by ultrasonography in asymptomatic adults. Man Ther 2007;12:286-94. [Crossref] [PubMed]
- Yalcin E, Akyuz M, Onder B, et al. Skin thickness on bony prominences measured by ultrasonography in patients with spinal cord injury. J Spinal Cord Med 2013;36:225-30. [Crossref] [PubMed]
- Sheikh K, Belfi LM, Sharma R, et al. Evaluation of acute cervical spine imaging based on ACR Appropriateness Criteria®. Emerg Radiol 2012;19:11-7. [Crossref] [PubMed]
- Sciubba DM, McLoughlin GS, Gokaslan ZL, et al. Are computed tomography scans adequate in assessing cervical spine pain following blunt trauma? Emerg Med J 2007;24:803-4. [Crossref] [PubMed]
- Ackland HM, Cameron PA, Varma DK, et al. Cervical spine magnetic resonance imaging in alert, neurologically intact trauma patients with persistent midline tenderness and negative computed tomography results. Ann Emerg Med 2011;58:521-30. [Crossref] [PubMed]
- Provoost V, Geusens E, Brys P, et al. A special case on the use of ultrasonography for evaluation of the spinal canal and its contents in adults. Emerg Radiol 2005;11:150-2. [Crossref] [PubMed]
- Shaw KA, Dougherty JJ, Treffer KD, et al. Establishing the content validity of palpatory examination for the assessment of the lumbar spine using ultrasonography: a pilot study. J Am Osteopath Assoc 2012;112:775-82. [Crossref] [PubMed]
- Vordemvenne T, Hartensuer R, Löhrer L, et al. Is there a way to diagnose spinal instability in acute burst fractures by performing ultrasound? Eur Spine J 2009;18:964-71. [Crossref] [PubMed]
- Prushansky T, Geller S, Avraham A, et al. Angular and linear spinal parameters associated with relaxed and erect postures in healthy subjects. Physiother Theory Pract 2013;29:249-57. [Crossref] [PubMed]
- Gercek E, Wahlen BM, Rommens PM. In vivo ultrasound real-time motion of the cervical spine during intubation under manual in-line stabilization: a comparison of intubation methods. Eur J Anaesthesiol 2008;25:29-36. [Crossref] [PubMed]
- Chleboun GS, Amway MJ, Hill JG, et al. Measurement of segmental lumbar spine flexion and extension using ultrasound imaging. J Orthop Sports Phys Ther 2012;42:880-5. [Crossref] [PubMed]
- Haque S, Bilal Shafi BB, Kaleem M. Imaging of torticollis in children. Radiographics 2012;32:557-71. [Crossref] [PubMed]
- Teng CC, Chai H, Lai DM, et al. Cervicocephalic kinesthetic sensibility in young and middle-aged adults with or without a history of mild neck pain. Man Ther 2007;12:22-8. [Crossref] [PubMed]
- Strimpakos N, Sakellari V, Gioftsos G, et al. Cervical spine ROM measurements: optimizing the testing protocol by using a 3D ultrasound-based motion analysis system. Cephalalgia 2005;25:1133-45. [Crossref] [PubMed]
- Zsidai A, Kocsis L. Ultrasound based measuring-diagnostic and muscle activity measuring system for spinal analysis. Technol Health Care 2006;14:243-50. [PubMed]
- Natarajan RN, Williams JR, Andersson GB. Modeling changes in intervertebral disc mechanics with degeneration. J Bone Joint Surg Am 2006;88 Suppl 2:36-40. [PubMed]
- Mueller LA, Degreif J, Schmidt R, et al. Ultrasound-guided spinal fracture repositioning, ligamentotaxis, and remodeling after thoracolumbar burst fractures. Spine (Phila Pa 1976) 2006;31:E739-46; discussion E747.
- Ledsome JR, Lessoway V, Susak LE, et al. Diurnal changes in lumbar intervertebral distance, measured using ultrasound. Spine (Phila Pa 1976) 1996;21:1671-5. [Crossref] [PubMed]
- Finlayson RJ, Etheridge JP, Tiyaprasertkul W, et al. A prospective validation of biplanar ultrasound imaging for C5-C6 cervical medial branch blocks. Reg Anesth Pain Med 2014;39:160-3. [Crossref] [PubMed]
- Kim D, Choi D, Kim C, et al. Transverse process and needles of medial branch block to facet joint as landmarks for ultrasound-guided selective nerve root block. Clin Orthop Surg 2013;5:44-8. [Crossref] [PubMed]
- Stiffler KA, Jwayyed S, Wilber ST, et al. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med 2007;25:331-4. [Crossref] [PubMed]
- Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth 2008;100:230-4. [Crossref] [PubMed]
- Halpern SH, Banerjee A, Stocche R, et al. The use of ultrasound for lumbar spinous process identification: A pilot study. Can J Anaesth 2010;57:817-22. [Crossref] [PubMed]
- Ungi T, Moult E, Schwab JH, et al. Tracked ultrasound snapshots in percutaneous pedicle screw placement navigation: a feasibility study. Clin Orthop Relat Res 2013;471:4047-55. [Crossref] [PubMed]
- Yamauchi M, Honma E, Mimura M, et al. Identification of the lumbar intervertebral level using ultrasound imaging in a post-laminectomy patient. J Anesth 2006;20:231-3. [Crossref] [PubMed]
- Lee AJ, Ranasinghe JS, Chehade JM, et al. Ultrasound assessment of the vertebral level of the intercristal line in pregnancy. Anesth Analg 2011;113:559-64. [PubMed]
- Lo MD, Parisi MT, Brown JC, et al. Sitting or tilt position for infant lumbar puncture does not increase ultrasound measurements of lumbar subarachnoid space width. Pediatr Emerg Care 2013;29:588-91. [Crossref] [PubMed]
- Ramsay N, Walker J, Tang R, et al. Flexion-rotation manoeuvre increases dimension of the acoustic target window for paramedian thoracic epidural access. Br J Anaesth 2014;112:556-62. [Crossref] [PubMed]
- Fanning N, Arzola C, Balki M, et al. Lumbar dural sac dimensions determined by ultrasound helps predict sensory block extent during combined spinal-epidural analgesia for labor. Reg Anesth Pain Med 2012;37:283-8. [Crossref] [PubMed]
- Avramescu S, Arzola C, Tharmaratnam U, et al. Sonoanatomy of the thoracic spine in adult volunteers. Reg Anesth Pain Med 2012;37:349-53. [Crossref] [PubMed]
- Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol 2010;60:13-9. [Crossref] [PubMed]
- Lee Y, Tanaka M, Carvalho JC. Sonoanatomy of the lumbar spine in patients with previous unintentional dural punctures during labor epidurals. Reg Anesth Pain Med 2008;33:266-70. [Crossref] [PubMed]
- Edelbauer M, Maurer K, Gassner I. Spinal subdural effusion - an additional sonographic sign of child abuse. Ultraschall Med 2012;33:E339-43. [Crossref] [PubMed]
- Yoon SH, O’Brien SL, Tran M. Ultrasound Guided Spine Injections : Advancement Over Fluoroscopic Guidance ? Current Science Inc 2013;1:104-13.
- Kim SH, Lee KH, Yoon KB, et al. Sonographic estimation of needle depth for cervical epidural blocks. Anesth Analg 2008;106:1542-7. table of contents. [Crossref] [PubMed]
- Rasoulian A, Lohser J, Najafi M, et al. Utility of prepuncture ultrasound for localization of the thoracic epidural space. Can J Anaesth 2011;58:815-23. [Crossref] [PubMed]
- Salman A, Arzola C, Tharmaratnam U, et al. Ultrasound imaging of the thoracic spine in paramedian sagittal oblique plane: the correlation between estimated and actual depth to the epidural space. Reg Anesth Pain Med 2011;36:542-7. [Crossref] [PubMed]
- Ozer Y, Ozer T, Altunkaya H, et al. The posterior lumbar dural depth: an ultrasonographic study in children. Agri 2005;17:53-7. [PubMed]
- Boezaart AP, Lucas SD, Elliott CE. Paravertebral block: cervical, thoracic, lumbar, and sacral. Curr Opin Anaesthesiol 2009;22:637-43. [Crossref] [PubMed]
- Perlas A. Evidence for the use of ultrasound in neuraxial blocks. Reg Anesth Pain Med 2010;35:S43-6. [Crossref] [PubMed]
- Herring AA, Stone MB, Frenkel O, et al. The ultrasound-guided superficial cervical plexus block for anesthesia and analgesia in emergency care settings. Am J Emerg Med 2012;30:1263-7. [Crossref] [PubMed]
- Ilfeld BM, Loland VJ, Mariano ER. Prepuncture ultrasound imaging to predict transverse process and lumbar plexus depth for psoas compartment block and perineural catheter insertion: a prospective, observational study. Anesth Analg 2010;110:1725-8. [Crossref] [PubMed]
- Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology 2011;115:94-101. [Crossref] [PubMed]
- Chin KJ, Perlas A. Ultrasonography of the lumbar spine for neuraxial and lumbar plexus blocks. Curr Opin Anaesthesiol 2011;24:567-72. [Crossref] [PubMed]
- Yamauchi M, Suzuki D, Niiya T, et al. Ultrasound-guided cervical nerve root block: spread of solution and clinical effect. Pain Med 2011;12:1190-5. [Crossref] [PubMed]
- Jee H, Lee JH, Kim J, et al. Ultrasound-guided selective nerve root block versus fluoroscopy-guided transforaminal block for the treatment of radicular pain in the lower cervical spine: a randomized, blinded, controlled study. Skeletal Radiol 2013;42:69-78. [Crossref] [PubMed]
- Chin KJ, Karmakar MK, Peng P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology 2011;114:1459-85. [Crossref] [PubMed]
- Gofeld M, Bristow SJ, Chiu SC, et al. Ultrasound-guided lumbar transforaminal injections: feasibility and validation study. Spine (Phila Pa 1976) 2012;37:808-12. [Crossref] [PubMed]
- Loizides A, Peer S, Plaikner M, et al. Ultrasound-guided injections in the lumbar spine. Med Ultrason 2011;13:54-8. [PubMed]
- Harries LW, Watura R. Septic arthritis of unilateral lumbar facet joint with contiguous abscess, without prior intervention. BMJ Case Rep 2012.2012. [PubMed]
- Galiano K, Obwegeser AA, Walch C, et al. Ultrasound-guided versus computed tomography-controlled facet joint injections in the lumbar spine: a prospective randomized clinical trial. Reg Anesth Pain Med 2007;32:317-22. [PubMed]
- Galiano K, Obwegeser AA, Bale R, et al. Ultrasound-guided and CT-navigation-assisted periradicular and facet joint injections in the lumbar and cervical spine: a new teaching tool to recognize the sonoanatomic pattern. Reg Anesth Pain Med 2007;32:254-7. [PubMed]
- Loizides A, Gruber H, Peer S, et al. Ultrasound guided versus CT-controlled pararadicular injections in the lumbar spine: a prospective randomized clinical trial. AJNR Am J Neuroradiol 2013;34:466-70. [Crossref] [PubMed]
- Obernauer J, Galiano K, Gruber H, et al. Ultrasound-guided versus Computed Tomography-controlled facet joint injections in the middle and lower cervical spine: a prospective randomized clinical trial. Med Ultrason 2013;15:10-5. [Crossref] [PubMed]
- Tacon CL, Flower O. Diagnosis and management of bacterial meningitis in the paediatric population: a review. Emerg Med Int 2012;2012. [Crossref] [PubMed]
- Duniec L, Nowakowski P, Kosson D, et al. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther 2013;45:1-6. [Crossref] [PubMed]
- Peterson MA, Pisupati D, Heyming TW, et al. Ultrasound for routine lumbar puncture. Acad Emerg Med 2014;21:130-6. [Crossref] [PubMed]
- Balki M, Lee Y, Halpern S, et al. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg 2009;108:1876-81. [Crossref] [PubMed]
- Chin KJ, Chan VW, Ramlogan R, et al. Real-time ultrasound-guided spinal anesthesia in patients with a challenging spinal anatomy: two case reports. Acta Anaesthesiol Scand 2010;54:252-5. [Crossref] [PubMed]
- Peterson MA, Abele J. Bedside ultrasound for difficult lumbar puncture. J Emerg Med 2005;28:197-200. [Crossref] [PubMed]
- Margarido C, Mikhael R, Salman A, et al. Epidural anesthesia for Cesarean delivery in a patient with post-traumatic cervical syringomyelia. Can J Anaesth 2011;58:764-8. [Crossref] [PubMed]
- Osterman MJ, Martin JA. National Vital Statistics Reports Epidural and Spinal Anesthesia Use During Labor: 27-State Reporting Area, 2008. Natl Vital Stat Rep 2011;59:1-13, 16.
- Arzola C, Davies S, Rofaeel A, et al. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg 2007;104:1188-92. tables of contents. [Crossref] [PubMed]
- Chin KJ, Macfarlane AJR, Chan V, et al. The use of ultrasound to facilitate spinal anesthesia in a patient with previous lumbar laminectomy and fusion: a case report. J Clin Ultrasound 2009;37:482-5. [Crossref] [PubMed]
- Balki M. Locating the epidural space in obstetric patients-ultrasound a useful tool: continuing professional development. Can J Anaesth 2010;57:1111-26. [Crossref] [PubMed]
- Lu IC, Huang SH, Hsu CD, et al. Lumbar epidural space was narrower in parturients than that in nonpregnant women by ultrasound assessment. Kaohsiung J Med Sci 2011;27:20-4. [Crossref] [PubMed]
- Amin WA, Abou Seada MO, Bedair E, et al. Comparative study between ultrasound determination and clinical assessment of the lumbar interspinous level for spinal anesthesia. Middle East J Anaesthesiol 2014;22:407-12. [PubMed]
- Tran D, Hor KW, Lessoway VA, et al. Adaptive ultrasound imaging of the lumbar spine for guidance of epidural anesthesia. Comput Med Imaging Graph 2009;33:593-601. [Crossref] [PubMed]
- Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth 2009;56:643-50. [Crossref] [PubMed]
- Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth 2010;57:313-21. [Crossref] [PubMed]
- Borges BC, Wieczorek P, Balki M, et al. Sonoanatomy of the lumbar spine of pregnant women at term. Reg Anesth Pain Med 2009;34:581-5. [Crossref] [PubMed]
- National Aeronautics and Space Administration. Technology Frontiers: Breakthrough Capabilities for Space Exploration. 2010:333. Available online: www.nasa.gov
- Margarido CB, Arzola C, Balki M, et al. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth 2010;57:120-6. [Crossref] [PubMed]
- Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth. Intensive Care 2014;42:480-6. [PubMed]
- Rihn JA, Fisher C, Harrop J, et al. Assessment of the Posterior Ligamentous Complex Following Acute Cervical Spine Trauma. J Bone Joint Surg Am 2010;92:583-9. [Crossref] [PubMed]


