Systematic review of cortical bone trajectory versus pedicle screw techniques for lumbosacral spine fusion
Introduction
Fusion of the lumbosacral spine is a common surgical procedure to address spinal pathologies including degenerative disk disease, lumbar stenosis, deformities, trauma, and neoplasms (1-3). Fixation in lumbar fusion necessitates the insertion of screws into the vertebrae. Traditionally, this has been done via pedicle screw (PS) augmentation of the posterior lumbosacral spine as first described by Boucher in 1959 (4). However, advances in spine surgery and a more general trend towards the adoption of less invasive procedures have led to the development of new and innovative techniques, which aim to achieve spinal fixation while causing less damage to surrounding tissues (5,6).
One of these newer methodologies is screw insertion via a cortical bone trajectory (CBT), initially described in spinal trauma as the medio-latero-superior technique (MLST) and later reintroduced by Santoni et al. in 2009 (7-10) as CBT. CBT is thought to have improved initial fixation by optimizing contact of the screw with the cortical bone of the vertebrae (11). Furthermore, the medial entry of the screw in CBT allows for minimal soft tissue dissection in comparison to traditional pedicle screws, and may reduce the risk of neurovascular injury (12). As such, CBT theoretically offers increased bone purchase with reduced invasiveness.
Since the introduction of CBT, a number of morphometric and biomechanical studies have supported its viability for pedicle fixation (11-18). Nevertheless, there is limited clinical evidence available in the literature that directly compares outcomes and complications between CBT and the traditional PS technique (8,11,15,19-23).
Methods
Purpose
The objective of this review is to summarize the literature regarding clinical investigations comparing CBT to PS, in order to assess needs for future research and elucidate differences in both operative outcomes and complications. This will be achieved through a systematic review, following recommended guidelines (24,25).
Search strategy and study selection
A comprehensive search of published reports was performed via six electronic databases; namely, Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials, Cochran Database of Systematic Reviews, American College of Physicians Journal Club and Database of Abstracts of Review of Effectiveness from their date of inception to [INSERT DATE]. To maximise the sensitivity of the search strategy, the terms; “cortical bone”, “cortical bone trajectory”, “CBT”, “medial-lateral superior trajectory”, “spine”, and “pedicle screw” were combined as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed to identify additional potentially relevant studies.
Eligible studies for the present systematic review included those in which cohorts undergoing CBT and PS procedures were directly compared. Studies that did not provide direct comparison of CBT and PS, or that did not report data on outcomes or complications were excluded. Abstracts, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Quality assessment
Studies were independently assessed for quality by two investigators (K Phan, RJ Mobbs) using the GRADE criteria (26). Discrepancies between the two reviewers were resolved by discussion and consensus.
Results
Included studies and quality of evidence
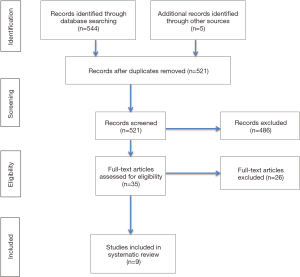
An extensive literature search identified nine relevant papers comparing CBT and PS methodologies for lumbosacral spine fusion (Figure 1) (27-35). All studies were classified as either retrospective cohort, prospective cohort, or case-control. The quality of evidence for all papers, using the GRADE criteria, was estimated to be low to medium based on the observational nature of the studies. More than half of the relevant studies had ≥40 patients with a third of all relevant studies exceeding 100 patients.
Baseline characteristics
Table 1 shows the baseline characteristics for each study that met inclusion criteria for comparison of CBT and PS including age, sex, diagnosis, vertebral level involvement, and average follow-up. Only one study, Chen et al., identified that there was no statistically significant difference between CBT and PS groups based on smoking status (27). Additionally, both Chin et al. and Takenaka et al. identified no difference in CBT and PS groups based on body mass index (28,35).
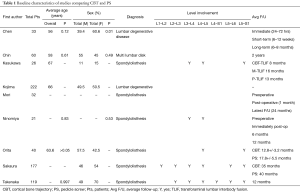
Full table
Intraoperative & postoperative outcomes and complications
Figure 2 shows an overall analysis of intraoperative outcomes, postoperative outcomes, and complications analyzed by all papers included in the systematic review. It delineates the specific findings of each study specifically comparing CBT to PS and highlights the significant gap that is present in the literature for commonly reported outcomes and complications.

Discussion
Previous studies
Alternative cortical trajectories for pedicle fixation in lumbar fusion have been proposed in clinical practice for over a decade. Steel et al. reported applying a medio-lateral superior trajectory for monosegmental screw fixation in patients with a thoracolumbar burst facture (8). After the initial description of CBT by Santoni et al. in 2009, several biomechanical studies were performed to evaluate the stability of the technique relative to traditional PS (7,12,16-18) although clinical evidence remained limited (15,19-23).
These authors previously reviewed the literature on the biomechanical, morphometric and clinical outcomes of CBT, and concluded that the available data were too few to allow for a definite evaluation of its merits; though CBT appeared to offer several advantages over the traditional PS approach (9). Several clinical studies have since been conducted that compare complications and outcomes between CBT and PS. A further review of the evidence is therefore warranted.
Techniques for CBT versus PS
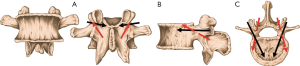
The traditional PS approach to lumbosacral spine surgery, currently the standard of care, requires extensive lateral spinal dissection for screw placement. In contrast, the CBT procedure requires less soft tissue exposure as screws are placed medially to laterally with a starting point at the junction between the lateral pars interarticularis and superior articular process (1 mm inferior to the inferior border of the transverse process, which was projected to the 5 o’clock orientation in the left pedicle and the 7 o’clock orientation in the right pedicle). Figure 3 shows the contrast in the trajectories of screw placement for both PS and CBT procedures in various planes (15). Recent trends have demonstrated a transition towards minimally invasive surgical approaches over traditional invasive approaches (16). Since CBT requires less dissection of the spine and smaller incisions than PS, it is considered to be the more minimally invasive of the two approaches.
Pain
With the exception of Ninomiya et al, pain was evaluated utilizing the visual analog scale (VAS) Pain score (32). Chen et al., Chin et al., Orita et al., Ninomiya et al., and Takenaka et al. all reported no significant differences in preoperative back or leg pain among their subjects (P>0.05) (27,28,32,33,35). Orita et al. and Ninomiya et al. saw a decrease in pain among both CBT and PS cohorts postoperatively. However, there was no significant difference between the CBT and PS cohorts at the 1-, 3-, 6-, or 12-month follow-up (32,33). Chen et al. described no difference in postoperative back pain immediately after surgery between the two cohorts, but noted that the CBT cohort had a significantly higher VAS pain score at the final 8-month follow-up (6.14 vs. 3.8, P=0.02) (27). Chin et al. also described no differences in postoperative back pain immediately after surgery between the two cohorts, but noted that the PS cohort had a significantly higher VAS Pain score at two-year postoperative follow-up. Additionally, no difference was elicited in leg pain postoperatively between the CBT and PS cohorts (P=0.169) (28).
Overall, the majority of literature suggests that the CBT technique results in similar or decreased postoperative pain compared to the more traditional PS technique. Lee et al. suggested that the decrease in immediate postoperative period associated with CBT technique was due to the smaller incision size, decreased disruption of muscle attachments and soft-tissue dissections. In their study, Lee et al. found that cortical screws were associated with lower immediate postoperative pain (within one week of surgery) compared to pedicle screws. However, long-term pain was similar in both groups (36).
Oswestry Disability Index (ODI) score
Disability was evaluated utilizing the ODI. Chin et al. reported no significant differences between the CBT and PS cohorts preoperatively (40.8 vs. 44.6, P=0.053). The CBT cohort was found to have a significant reduction in ODI score compared to the PS cohort postoperatively (28.7 vs. 32.5 respectively, P=0.027). This was attributed to the ability to preserve anatomy, decreased operative time, decreased dissection, intraoperative blood loss, and less postoperative pain (28).
Complications
Complications were described extensively in Sakaura et al. and Takenaka et al. Intraoperatively, Sakaura et al. and Takenaka et al. described no significant differences in misplacement of screws among the two cohorts, with Sakaura et al. specifically reporting 2.1% in CBT placement and 3.7% in PS placement (34,35). It is likely that misplacement of screws is due more to surgical technique rather than approach. Kuo et al. showed that with the help of radiographic guidance, the accuracy of placement of lumbar screws was determined to be 94% before repositioning and 98.74% after repositioning (37).
Postoperatively, Sakaura et al. and Takenaka et al. described no significant differences with dural tears, symptomatic hematomas, superficial wound infections, or deep wound infections among the CBT and PS cohorts. Both saw increased incidence of dural tears among the PS group (3.7% and 2.1% respectively). However, this was not found to be statistically significant. With regards to the incidence of symptomatic hematoma, Takenaka et al. reported a higher incidence in the CBT cohort (2.4% vs. 0%) while Sakaura et al. reported similar incidences among both groups (1.1% vs. 1.2%). Both studies found the incidence of symptomatic hematoma to be similar between CBT and PS cohorts. With regards to rates of wound infection, both Sakaura et al. and Takenaka et al. found no significant differences between the rates of superficial or deep wound infections in either group (P>0.05). However, Sakaura et al. did notice a higher incidence of superficial wound infection in the CBT cohort (2.1% vs. 0%), and both papers noted a higher incidence of deep wound infection in the PS cohort (34,35). Hegde et al. suggests that rate of infection can be correlated to length and complexity of surgical procedures. The increased duration of surgery, dissection, and postoperative dead space are factors that may explain this finding (38).
Operative time
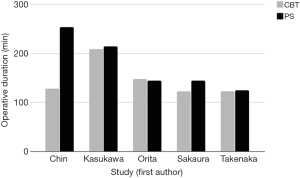
Operative time is an important consideration in assessing different techniques as increased operative duration may result in higher rates of infection, post-operative complications, and intra-operative outcomes such as increased blood loss (39-41). Several studies have investigated operative time in lumbar fusion procedures utilizing CBT and PS techniques. Three studies (Chin et al., Sakaura et al., and Takenaka et al.) directly compared operative times for CBT and PS procedures. Of these three studies, only Sakaura et al. demonstrated significant findings in that PS procedures were longer in duration (145±33 min) than lumbar fusions performed via CBT (123±16 min) (P<0.01). The other two studies, Chin et al. (P=0.084) and Takenaka et al. (P=0.672), showed no difference in operative time between the procedures (28,34,35). Orita et al. showed that percutaneously-performed CBT (147.3±23.3 min) and PS (144±19.2 min) did not vary in operative time (P=0.252) (33). Kasukawa et al. compared operative times of CBT (209±49 min) to PS inserted via conventional minimally invasive (198±51 min) or percutaneous methods (243±26 min). None of these values were statistically different when compared and the mean PS time between the two techniques was 214.9 minutes (29). Overall, operative times between the procedures did not vary significantly. In a wide variety of surgical fields, proper surgical technique has been associated with improved patient outcomes (42,43). This is true for spine surgery as well (44). As such, in deciding between CBT and PS, operative time may not be as important of a criterion in selection of an approach compared to a surgeon’s comfort with the technique and other factors that may portend patient outcomes in the peri-operative period. Figure 4 shows the complete comparison between intraoperative times of CBT and PS in five different studies.
Blood loss
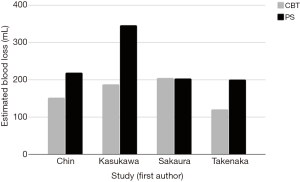
Blood loss is an important consideration in comparing different surgical techniques due to its impact on post-operative mortality (45). Analysis of the literature revealed four studies reporting blood loss for CBT and PS procedures. Chin et al. demonstrated that CBT resulted in less blood loss (152 mL) compared to PS (219 mL) (P<0.05) (28). Similarly, Takenaka et al. showed that CBT (120 mL) had less blood loss than PS (201 mL) (P<0.001) (35). Kasukawa delineated blood loss in PS procedures by those performed percutaneously (210±114 mL) and those performed via conventional minimally invasive techniques (429±289 mL), the weighted mean of which is represented in Figure 5. Their investigation showed that intraoperative blood loss was significantly less with CBT (188±167) compared to the conventional minimally invasive PS approach (29). Sakaura et al. showed no difference between intraoperative blood loss in CBT (205 mL) and PS (204 mL) procedures (34). Overall, these findings show that the CBT approach may yield less blood loss than PS approaches and may be an important consideration in determining surgical approach to lumbar fusion in patients who are higher risk surgical candidates. Additionally, it may be of importance in patients which chronic medical conditions such as chronic kidney disease and cardiac failure in whom anemia is commonplace or hemodynamic stability precipitated by blood loss may lead to adverse post-operative outcomes (46).
Japanese Orthopedic Association (JOA) Score
Some clinical research studies investigating lumbar fusion have utilized the JOA scale scoring system to assess the results of treatment for low-back pain. In the normal population, the total JOA score is a full 29 points. Specifically, the JOA evaluates symptoms, functionality, and clinical signs of low-back pain and may be used in pre- and post-operative evaluation of patients (47). Mori et al.showed that mean post-operative (25±2.0, P<0.001) and latest follow-up JOA scores (25±1.8, P<0.001) were significantly increased from mean pre-operative scores (12±4.9) in patients who underwent CBT (31). Sakaura et al. investigated JOA scores in patients who underwent CBT or PS. In the CBT cohort, pre-operative (13.7±4.6) and post-operative (23.3±4.71) JOA scores were significantly improved when compared (P<0.001). Similarly, in the group undergoing PS, pre-operative (14.4±3.9) and post-operative (22.7±3.71) JOA scores were improved significantly as well. While pre-operative scores between the cohorts were not significantly different, the recovery rate of JOA scores in the CBT group (64.4%±25.9%) was significantly higher than in the PS group (55.8%±26.4%) (P<0.05) (34).
Both CBT and PS greatly improve post-operative JOA scores, indicating improvement in patient overall quality of life from low-back pain. However, comparisons are lacking between CBT and PS to elucidate these techniques’ impact on patients’ self-reported outcomes. Prior low back pain research has revealed that patient-reported outcome indices such as the pain self-efficacy questionnaire (PSEQ) and the patient-specific functional scale (PSFS) are the most responsive scales in measuring changes in patients with chronic low back pain following a back class exercise regimen (48). By incorporating such scales in CBT and PS studies, self-reported patient outcomes may be better recorded. This may allow for enhanced selection of surgical technique based not only on quantitative surgical peri-operative measures, but also based on patient-reported measures which may be of equal importance.
Radiographic analysis of outcomes
Radiographic analysis of outcomes of CBT and PS can be used to assess fixation and bone density at the sites of fusion, providing another methodology to assess outcomes in these procedures. Ninomiya et al. showed that both CBT and PS result in a statistically significant decrease in percentage slippage for patients with lumbar degenerative spondylolisthesis immediately post-operatively, 6 months post-operatively, and one year post-operatively (P<0.05). Additionally, there was no significant loss of slippage percentage in CBT and PS at 6- and 12-month follow-up. However, neither CBT nor PS changed lumbar lordosis significantly at one-year postoperative follow-up (32). These findings denote that both CBT and PS are highly effective in adequately addressing the slippage percentage in patients with lumbar spondylolisthesis. However, further research is required to address residual lordosis and corroborate findings.
In another radiologic analysis, Kojima et al. utilized computerized tomography (CT) to assess pedicle screw-cortical bone contact between CBT and PS implanted screws. The investigation had several findings. There is a statistically significant difference in the Hounsfield units (HU) (a CT number used as a measure of bone density) between individuals >70-year-old and <70-year-old for both CBT and PS (P<0.001) at L4 and L5. Additionally, males have higher HU than females at L4 and L5 at baseline. In comparing CBT performed at L4 (726.7±13.61) and L5 (711.4±20.58), there was no difference in HU. The same was found for PS performed at L4 (171.3±4.01) and L5 (173.3±4.88). However, there is a statistically significant difference in HU between CBT and PS at L4 (P<0.0001) and L5 (P<0.0001) (30). These findings suggest that CBT results in richer cortical bone at the site of screw placement, perhaps alluding to its utility for anchoring pedicle to the vertebrae. This is especially pertinent when performing procedures on older patients, who often have diminished bone density. In fact, the average age of the patient population undergoing lumbar fusion has been increasing, heightening the importance of considering techniques with increased cortical bone purchase (49).
Conclusions
There is no widely accepted consensus regarding comparison of outcomes and complications between the CBT and PS procedures. Generally, indications for CBT and PS are similar between most studies, especially for common pathologies resulting in spondylolisthesis. In terms of intraoperative outcomes, most studies found no significant differences between operative time, but found that CBT has less blood loss than PS. Post-operative outcomes including pain have inconclusive results as some studies found more post-operative pain for CBT while others found more post-operative pain with PS. Radiologic studies show no difference in slippage within one-year of follow-up after CBT and PS, but there is a significant difference in resulting bone density post-procedure for CBT when compared to PS, which has tremendous implications on stability for lumbosacral spine surgeries in elderly patients. Future studies, including randomized controlled trials, would control for more confounding factors and offer stronger evidence towards choosing CBT versus PS. The current literature reveals significant gaps in measurement of both patient-reported outcomes and quality metrics that impact the decision of both patients and surgeons when evaluating the benefits and risks of both CBT and PS procedures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kaiser MG, Eck JC, Groff MW, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine 2014;21:2-6. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Wu AM, Zou F, Cao Y, et al. Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment. AME Med J 2017;2:63. [Crossref]
- Boucher HH. A method of spinal fusion. J Bone Joint Surg Br 1959;41:248-59. [PubMed]
- Lowery GL, Kulkarni SS. Posterior percutaneous spine instrumentation. Eur Spine J 2000;9:S126-30. [Crossref] [PubMed]
- Song T, Hsu WK, Ye T. Lumbar pedicle cortical bone trajectory screw. Chin Med J 2014;127:3808-13. [PubMed]
- Santoni B, Hynes R, McGilvray K, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009;9:366-73. [Crossref] [PubMed]
- Steel T, Rust T, Fairhall J, et al., editors. Monosegmental pedicle screw fixation for thoraco-lumbar burst fracture. In: Orthopaedic Proceedings. Orthopaedic Proceedings 2004:458.
- Phan K, Hogan J, Maharaj M, et al. Cortical bone trajectory for lumbar pedicle screw placement: a review of published reports. Orthop Surg 2015;7:213-21. [Crossref] [PubMed]
- Mobbs RJ. The “Medio-Latero-Superior Trajectory Technique”: an alternative cortical trajectory for pedicle fixation. Orthop Surg 2013;5:56-9. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Kato T, et al. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine 2014;39:E240-5. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Nemoto O, et al. Morphometric measurement of cortical bone trajectory for lumbar pedicle screw insertion using computed tomography. J Spinal Disord Tech 2013;26:E248-53. [Crossref] [PubMed]
- Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48. [Crossref] [PubMed]
- Weinstein JN, Rydevik BL, Rauschning W. Anatomic and technical considerations of pedicle screw fixation. Clin Orthop Relat Res 1992.34-46. [PubMed]
- Iwatsuki K, Yoshimine T, Ohnishi Y, et al. Isthmus-guided cortical bone trajectory for pedicle screw insertion. Orthop Surg 2014;6:244-8. [Crossref] [PubMed]
- Perez-Orribo L, Kalb S, Reyes PM, et al. Biomechanics of lumbar cortical screw–rod fixation versus pedicle screw–rod fixation with and without interbody support. Spine 2013;38:635-41. [Crossref] [PubMed]
- Baluch DA, Patel AA, Lullo B, et al. Effect of physiological loads on cortical and traditional pedicle screw fixation. Spine 2014;39:E1297-302. [Crossref] [PubMed]
- Calvert GC, Lawrence BD, Abtahi AM, et al. Cortical screws used to rescue failed lumbar pedicle screw construct: a biomechanical analysis. J Neurosurg Spine 2015;22:166-72. [Crossref] [PubMed]
- Ueno M, Imura T, Inoue G, et al. Posterior corrective fusion using a double-trajectory technique (cortical bone trajectory combined with traditional trajectory) for degenerative lumbar scoliosis with osteoporosis: Technical note. J Neurosurg Spine 2013;19:600-7. [Crossref] [PubMed]
- Gonchar I, Kotani Y, Matsui Y, et al. Experience of 100 consecutive spine reconstructions using cortical bone trajectory (CBT) screws vs. traditional pedicle screws. Proceeding of SMISS Global Forum 2014; 2014 Sep 19-21; Miami, FL, USA.
- Gonchar I, Kotani Y, Matsumoto Y. Cortical bone trajectory versus percutaneous pedicle screw in minimally invasive posterior lumbar fusion. Spine J 2014;14:S114-5. [Crossref]
- Rodriguez A, Neal MT, Liu A, et al. Novel placement of cortical bone trajectory screws in previously instrumented pedicles for adjacent-segment lumbar disease using CT image-guided navigation. Neurosurg Focus 2014;36:E9. [Crossref] [PubMed]
- Takata Y, Matsuura T, Higashino K, et al. Hybrid technique of cortical bone trajectory and pedicle screwing for minimally invasive spine reconstruction surgery: a technical note. J Med Invest 2014;61:388-92. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg 2015;1:19-27. [PubMed]
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. [Crossref] [PubMed]
- Chen YR, Deb S, Pham L, et al. Minimally invasive lumbar pedicle screw fixation using cortical bone trajectory–a prospective cohort study on postoperative pain outcomes. Cureus 2016;8:e714. [PubMed]
- Chin KR, Pencle FJ, Coombs AV, et al. Clinical outcomes with midline cortical bone trajectory pedicle screws versus traditional pedicle screws in moving lumbar fusions from hospitals to outpatient surgery centers. Clin Spine Surg 2017;30:E791-7. [PubMed]
- Kasukawa Y, Miyakoshi N, Hongo M, et al. Short-term results of transforaminal lumbar interbody fusion using pedicle screw with cortical bone trajectory compared with conventional trajectory. Asian Spine J 2015;9:440-8. [Crossref] [PubMed]
- Kojima K, Asamoto S, Kobayashi Y, et al. Cortical bone trajectory and traditional trajectory—a radiological evaluation of screw-bone contact. Acta Neurochir (Wien) 2015;157:1173-8. [Crossref] [PubMed]
- Mori K, Nishizawa K, Nakamura A, et al. Short-term clinical result of cortical bone trajectory technique for the treatment of degenerative lumbar spondylolisthesis with more than 1-year follow-up. Asian Spine J 2016;10:238-44. [Crossref] [PubMed]
- Ninomiya K, Iwatsuki K, Ohnishi YI, et al. Radiological evaluation of the initial fixation between cortical bone trajectory and conventional pedicle screw technique for lumbar degenerative spondylolisthesis. Asian Spine J 2016;10:251-7. [Crossref] [PubMed]
- Orita S, Inage K, Kubota G, et al. One-year prospective evaluation of the technique of percutaneous cortical bone trajectory spondylodesis in comparison with percutaneous pedicle screw fixation: a preliminary report with technical note. J Neurol Surg A Cent Eur Neurosurg 2016;77:531-7. [Crossref] [PubMed]
- Sakaura H, Miwa T, Yamashita T, et al. Posterior lumbar interbody fusion with cortical bone trajectory screw fixation versus posterior lumbar interbody fusion using traditional pedicle screw fixation for degenerative lumbar spondylolisthesis: a comparative study. J Neurosurg Spine 2016;25:591-5. [Crossref] [PubMed]
- Takenaka S, Mukai Y, Tateishi K, et al. Clinical outcomes after posterior lumbar interbody fusion: comparison of cortical bone trajectory and conventional pedicle screw insertion. Clin Spine Surg 2017. [Epub ahead of print]. [PubMed]
- Lee GW, Son JH, Ahn MW, et al. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J 2015;15:1519-26. [Crossref] [PubMed]
- Kuo KL, Su YF, Wu CH, et al. Assessing the intraoperative accuracy of pedicle screw placement by using a bone-mounted miniature robot system through secondary registration. PloS One 2016;11:e0153235. [Crossref] [PubMed]
- Hegde V, Meredith DS, Kepler CK, et al. Management of postoperative spinal infections. World J Orthop 2012;3:182-9. [Crossref] [PubMed]
- Zheng F, Cammisa FP Jr, Sandhu HS, et al. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine 2002;27:818-24. [Crossref] [PubMed]
- Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg 2015;220:550-8. [Crossref] [PubMed]
- Peersman G, Laskin R, Davis J, et al. Prolonged operative time correlates with increased infection rate after total knee arthroplasty. HSS J 2006;2:70-2. [Crossref] [PubMed]
- Hermanek P. Impact of surgeon's technique on outcome after treatment of rectal carcinoma. Dis Colon Rectum 1999;42:559-62. [Crossref] [PubMed]
- Birkmeyer JD, Finks JF, O'reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434-42. [Crossref] [PubMed]
- Mannion AF, Elfering A. Predictors of surgical outcome and their assessment. Eur Spine J 2006;15 Suppl:S93-108. [Crossref] [PubMed]
- Zou H, Li Z, Sheng H, et al. Intraoperative blood loss, postoperative drainage, and recovery in patients undergoing lumbar spinal surgery. BMC Surg 2015;15:76. [Crossref] [PubMed]
- Acedillo RR, Shah M, Devereaux P, et al. The risk of perioperative bleeding in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Surg 2013;258:901-13. [Crossref] [PubMed]
- Yone K, Sakou T, Kawauchi Y, et al. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine 1996;21:242-8. [Crossref] [PubMed]
- Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J 2010;19:1484-94. [Crossref] [PubMed]
- Verla T, Adogwa O, Toche U, et al. Impact of increasing age on outcomes of spinal fusion in adult idiopathic scoliosis. World Neurosurg 2016;87:591-7. [Crossref] [PubMed]