
Ruminococcus gnavus, an unusual cause of surgical site infection following vertebral posterior instrumentation: a case report
Introduction
Surgical site infection (SSI) following spine surgery can occur in 0.7% to 11.9% of patients with significant morbidity and economic burden (1,2). Staphylococcus aureus is the most frequent causal agent (3). Diabetes, smoking, steroids, surgical invasiveness, approach, duration of the procedure, type of fusion, implant use… are some of the main risk factors related to SSI. Malnutrition has been identified as an important and preventable risk factor for the development of SSI. Specifically, having a Modified Glasgow Prognostic Scale (mGPS) score of 1 or more or a low body mass index (BMI <20.39 kg/m2) have been identified as risk factors for developing SSI after spinal instrumentation surgery (4). Early diagnosis of SSI is of vital importance to reduce morbidity costs and also to allow early treatment. Some inflammatory markers have been identified as particularly useful for early diagnosis after psoterior lumbar surgery: white blood cells (WBCs) count and their subsets, lymphocites counts on postoperative day (POD) 4 and 7, C-reactive protein (CRP) levels on the 7th day after surgery and the neutrophil/lymphocyte count ratio (NLR) (5).
Anaerobic organisms as etiologic agents of bone and BJI are relatively infrequent and their incidence is estimated to be around 3% to 4% of all BJI (6). Moreover, limitations in our current conventional culture-based testing make it difficult to isolate anaerobic organisms, so their incidence is frequently underestimated. New technologies such as mass spectrometry techniques and molecular diagnostic technology, such as 16S rRNA may lead to greater recognition of anaerobic bacteria and their involvement in BJI. Propionibacterium acnes is the most prevalent causal agent in BJI, Bacteroides spp., Clostridium spp., Finegoldia magna, or Peptoniphilus spp have also been reported (6). In relation to treatment, clindamycin is often the treatment of choice for anaerobic infections, not only because of its ability to penetrate the bone, but also due to its action against both, Gram positive and negative anaerobic bacteria. Amoxicilin and metronidazole are also recommended (6,7).
We present the first case report of deep SSI following spinal surgery due to R. Gnavus, a strict anaerobic Gram-positive coccus. We present the following case in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-22-81/rc).
Case presentation
Statement of informed consent
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Case description
The patient first came for consultation in 2020 at the age of 79 years old. She presented with chronic low back pain radiating to groin and anterior region of left thigh and leg. Her medical history included hyperlipidemia, diabetes mellitus, atrophic gastritis and polymyalgia rheumatica. Regarding her nutritional status, her BMI was 27.2 kg/m2 and serum proteins in the preoperative study were in the normal range (6.9 g/dL).
The magnetic resonance imaging (MRI) showed a voluminous L2-L3 disc herniation and advanced degenerative changes at L3-L4 and L4-L5. She underwent a L2-L3 discectomy and posterior L2-L5 fusion. No complications were recorded during postoperative evolution. Nine months after, the patient presented with worsening back pain, no significant neurological findings on examination. The X-ray showed an adjacent segment disease (ASD) and scoliosis just proximal to the instrumentation. Standing radiographs revealed ASD, as an apex right curve just proximal to the instrumentation (Figure 1) and positive sagittal imbalance of 12 cm. The MRI showed no evidence of further complication.
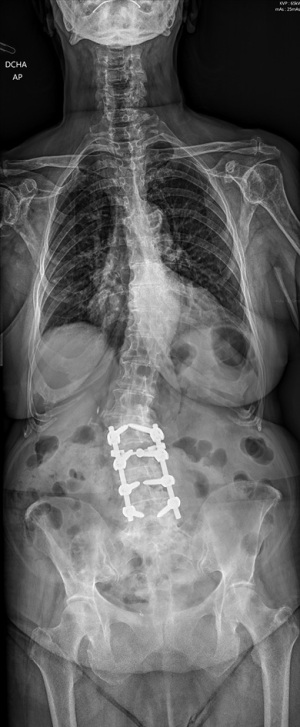
In 2021 at the age of 81, the patient underwent extension of previous fusion from T10 to S2-alar-iliac (Xia 3 System Serrato Stryker), an asymmetric transforaminal interbody device was placed at L1-L2, in situ scoliotic correction was performed as well as rod derotation. At levels T10 and T11 cement-augmented screws were used and proximal to the instrumentation, at T9, we performed a prophylactic vertebroplasty. Cancellous allograft in conjunction with local autograft were placed at L1-L2 and L5-S1 levels (Figure 2). We used intraoperative neurophysiology monitoring. Two subfascial drains were used. A dose of cefazolin 1 g was administered preoperatively, the dose was repeated 3 h after the start of surgery. During the postoperative period, 3 more doses of cefazolin 1 g were administered every 8 h. Operative time was 7 h 45 min, the patient received two units of red blood cells (RBC) postoperative.
On POD 4, the patient presented with abdominal pain and vomiting. With the suspicion of paralytic ileus in probable relation to opioid consumption, enteral nutrition was indicated and a nasogastric tube was placed. In spite of the nasogastric tube and the suspension of opioids the patient got worse, so on POD 13 a gastroscopy was performed in which a longitudinal gastric ulcer Forrest III was described, hemoclips were placed. On POD 14 the patient presented an episode of gastrointestinal bleeding and hematemesis with secondary anemization (hemoglobin 7 g/dL). A new upper gastrointestinal endoscopy was performed in which normopositioned hemoclips were observed and no active bleeding. Given the possibility that the ulcer was due to the decubitus of the nasogastric tube, we decided to remove it.
The surgical wound evolved favorably until day 17, when it began with seropurulent exudate in the distal region accompanied by leukocytosis and elevated infection markers, leukocytes 12.6×109/L, and the CRP level was 71 mg/L. No blood culture was collected, since the patient did not show fever at any time during the follow-up.
On POD 20 the patient underwent surgical irrigation and debridement, purulent-appearing fluid was encountered within the subcutaneous tissues and deep to the fascia. Exudate, soft tissue and bone samples were sent to microbiology. Thorough debridement of the area was performed, saline and povidone-iodine irrigation was done. One gram of vancomycin powder was applied directly to the subcutaneous tissue just prior to closure. Vancomycin powder was sprinkled liberally over the area. Empirical antibiotic therapy was administered with meropenem 1 g and teicoplanin 600 mg according to the usual protocol for musculoskeletal infections in our center.
Six out of six cultures on anaerobic conditions revealed short chains of Gram-positive diplococci. All the strains isolated from the different samples were identified as R. Gnavus by Maldi-TOF MS (Compass 1.4, version 3.4, build 3.4.76.0 by Bruker Daltonics) with score values ranging between 1.820 and 2.100. In addition, one of the samples yielded positive for a single colony, fully sensitive Escherichia coli (E. Coli), we considered it as a possible contaminant.
Susceptibility testing of R. Gnavus was performed using a commercial broth microdilution method (SensititreTM ANAERO3, Thermo Scientific) and showed the following results: penicillin (≤0.06 µ/mL), amoxicillin (≤0.25 µ/mL), amoxicillin/clavulanate (≤0.25/0.125 µ/mL), piperacillin (≤16 µ/mL), piperacillin/tazobactam (≤16/4 µ/mL), cefoxitin (8 µ/mL), imipenem (≤0.06 µ/mL), chloramphenicol (≤2 µ/mL), erythromycin (>128 µ/mL), clindamycin (1 µ/mL), metronidazole (≤0.5 µ/mL), moxifloxacin (8 µ/mL), tetracycline (8 µ/mL), and vancomycin (≤2 µ/mL). Moreover, E-test method (bioMérieux, Marcy l’Etoile, France) was used for other antimicrobials and the following results were obtained: linezolid (2 µ/mL), levofloxacin (>32 µ/mL), daptomycin (0.047 µ/mL), cotrimoxazole (>32 µ/mL), and rifampicin (<0.002 µ/mL). Penicillin, amoxicillin, amoxicillin/clavulanate, piperacillin, piperacillin/tazobactam, imipenem, chloramphenicol, clindamycin, and metronidazole minimum inhibitory concentration (MIC) breakpoints were those established by European Committee on Antimicrobial Susceptibility Testing (EUCAST) (8). The breakpoint MICs for the rest of antibiotics were established according to Clinical and Laboratory Standards Institute (CLSI) documents for anaerobic bacteria and other sources (7). According to these criteria, all antimicrobials tested were active against R. Gnavus except for erythromycin, moxifloxacin, levofloxacin, and cotrimoxazole.
After obtaining microbiological results, antibiotics were switched to amoxicilin 1 g/8 h and ciprofloxacin 750 mg/12 h for a further 4 weeks. Given the good clinical outcome, the patient was discharged on POD 29 after first surgery. On the patient’s last assessment, 6 months after surgery, she was asymptomatic, she had no back pain, she showed no sign of surgical wound infection and inflammatory markers were normal.
Discussion
R. Gnavus is a strict anaerobic Gram-positive coccus. It can be motile or nonmotile, non-spore-forming. Gram staining shows them as diplococci or short chains. It belongs to the Clostridia class. It is commonly found in the intestinal flora in humans and ruminant animals. In humans, R. Gnavus is among the 15 most frequent species in the microbiota (9). Several lines of evidence have shown an increase in the presence of R. Gnavus in patients suffering from Crohn’s disease or ulcerative colitis, suggesting R. Gnavus might have an important role in modulating inflammatory activity in the bowel (10).
As we mentioned above, anaerobic bacteria identification is often difficult given limitations in our current conventional culture-based testing. With the introduction of MALDI-TOF MS as a routine technique, anaerobic bacteria identification has improved considerably. Bacterial proteins are ionised by a laser beam, then we elaborate a unique protein spectrum for each bacterium (11). Subsequently, we compare the spectrum obtained with those collected in previous databases. Depending on the degree of concordance we will make the diagnosis. A score higher than 2 is a sure identification of the species, scores between 1.7 and 2 are a sure identification of the genus. In our hospital we use this technique routinely for the identification of anaerobic bacteria. In our patient MALDI-TOF MS revealed R. Gnavus presence in 6 out of 6 intra-operative samples.
Additionally, partial 16S rRNA gene sequencing can be useful as a confirmation of MALDI-TOF MS or as an alternative when MALDI-TOF MS does not provide identification. The samples were not saved and we do not routinely perform 16S sequencing if MALDI-TOF MS is conclusive.
R. Gnavus has been described previously in few case reports. Two cases of polymicrobial bacteriemia associated with diverticulitis (12), bacteriemia in a patient with a gall bladder perforation (13), sepsis in a patient suffering from multiple myeloma (14), sepsis due to bowel perforation (15) and 4 cases of BJI: a case of septic arthritis in a native hip (16) and 3 periprosthetic hip jont infections (17-19). In all cases the patients had a previous history of intestinal disease or immunosuppression. Previously published cases are listed in Table 1.
Table 1
| Study | Year | Country | Study design | Age (years) | Infection | Microorganisms isolated | R. Gnavus identification | Possible cause | Treatment and outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Hansen (12) | 2013 | Denmark | Case report | 67 | Bacteriemia | E. Coli | MALDI-TOF | Diverticular disease | Exploratory laparotomy |
| Perforated peritonitis | R. Gnavus | 16S rRNA | Lung carcinoma receiving chemotherapy | Piperacillin tazobactam | |||||
| He died one week later | |||||||||
| 90 | Bacteriemia | P. aeruginosa | MALDI-TOF | Diverticular disease | Cefuroxime and metronidazole | ||||
| R. Gnavus | 16S rRNA | Complete recovery | |||||||
| Kim (13) | 2017 | South Korea | Case report | 82 | Bacteriemia | R. Gnavus | MALDI-TOF | Gall bladder perforation | Antibiotics |
| 16S rRNA | DM | Asymptomatic | |||||||
| Gren (14) | 2019 | Denmark | Case report | 76 | Bacteriemia | R. Gnavus | MALDI-TOF | Multiple myeloma | Piperacillin |
| Myelodysplastic síndrome | Tazobactam | ||||||||
| Immunosupression | Amoxicilin | ||||||||
| Metronidazole | |||||||||
| Lefever (15) | 2019 | Belgium | Case report | 66 | Bacteriemia | E. Coli | 16S rRNA | Infectious enterocolitis | Levofloxacin |
| Fecal peritonitis | R. Gnavus | Ornidazol | |||||||
| Meropenem | |||||||||
| Critical illness polyneuropathy | |||||||||
| Titécat (16) | 2014 | France | Case report | 47 | Septic arthritis | R. Gnavus | MALDI-TOF | Squamous cell carcinoma | Vancomycin |
| Bacteriemia | 16S rRNA | Immunosupression | Cefotaxime | ||||||
| She died 4 weeks later of pneumonia | |||||||||
| Fernández-Caso (17) | 2017 | Spain | Case report | 72 | Total hip arthroplasty late infection | R. Gnavus | MALDI-TOF | None | Replacement surgery |
| Cefazolin | |||||||||
| Clindamycin | |||||||||
| Gentamycin | |||||||||
| Complete recovery | |||||||||
| Roux (18) | 2015 | France | Case report | 62 | Total hip arthroplasty late infection | R. Gnavus | MALDI-TOF | Ulcerative colitis | Irrigation and debridement |
| 16S rRNA | Amoxicilin | ||||||||
| Metronidazole | |||||||||
| Complete recovery | |||||||||
| Arnáez Solís (19) | 2017 | Spain | Case report | 93 | Total hip arthroplasty late infection | R. Gnavus | MALDI-TOF | DM | Replacement surgery |
| Immunosupression | She died two weeks after |
R. Gnavus, Ruminococcus gnavus; MALDI-TOF, matrix-assisted laser desorption/ionization time of flight; DM, diabetes mellitus.
To our knowledge, R. Gnavus has not been previously described in SSI after spinal surgery. We believe that the patient presented a hematogenous infection due to translocation from the gut, after gastrointestinal manipulation.
As previously pointed out by other authors, the isolation of R. Gnavus should lead us to consider the possibility of intestinal injury. Immunosuppression appears to be another important risk factor. Our patient was not immunosuppressed perse, however the surgical aggression may have been an immunocompromising event that favored infection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-22-81/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-22-81/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-22-81/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study studies were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dowdell J, Brochin R, Kim J, et al. Postoperative Spine Infection: Diagnosis and Management. Global Spine J 2018;8:37S-43S. [Crossref] [PubMed]
- Chaichana KL, Bydon M, Santiago-Dieppa DR, et al. Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. J Neurosurg Spine 2014;20:45-52. [Crossref] [PubMed]
- White AJ, Fiani B, Jarrah R, et al. Surgical Site Infection Prophylaxis and Wound Management in Spine Surgery. Asian Spine J 2022;16:451-61. [Crossref] [PubMed]
- Kobayashi Y, Inose H, Ushio S, et al. Body Mass Index and Modified Glasgow Prognostic Score Are Useful Predictors of Surgical Site Infection After Spinal Instrumentation Surgery: A Consecutive Series. Spine (Phila Pa 1976) 2020;45:E148-54. [Crossref] [PubMed]
- Shen CJ, Miao T, Wang ZF, et al. Predictive value of post-operative neutrophil/lymphocyte count ratio for surgical site infection in patients following posterior lumbar spinal surgery. Int Immunopharmacol 2019;74:105705. [Crossref] [PubMed]
- Walter G, Vernier M, Pinelli PO, et al. Bone and joint infections due to anaerobic bacteria: an analysis of 61 cases and review of the literature. Eur J Clin Microbiol Infect Dis 2014;33:1355-64. [Crossref] [PubMed]
- CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. 9th edition. CLSI standard M11. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 2018.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0, 2022.
- Hattori M, Taylor TD. The human intestinal microbiome: a new frontier of human biology. DNA Res 2009;16:1-12. [Crossref] [PubMed]
- Hall AB, Yassour M, Sauk J, et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med 2017;9:103. [Crossref] [PubMed]
- Lagacé-Wiens PR, Adam HJ, Karlowsky JA, et al. Identification of blood culture isolates directly from positive blood cultures by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry and a commercial extraction system: analysis of performance, cost, and turnaround time. J Clin Microbiol 2012;50:3324-8. [Crossref] [PubMed]
- Hansen SG, Skov MN, Justesen US. Two cases of Ruminococcus gnavus bacteremia associated with diverticulitis. J Clin Microbiol 2013;51:1334-6. [Crossref] [PubMed]
- Kim YJ, Kang HY, Han Y, et al. A bloodstream infection by Ruminococcus gnavus in a patient with a gall bladder perforation. Anaerobe 2017;47:129-31. [Crossref] [PubMed]
- Gren C, Spiegelhauer MR, Rotbain EC, et al. Ruminococcus gnavus bacteraemia in a patient with multiple haematological malignancies. Access Microbiol 2019;1:e000048. [Crossref] [PubMed]
- Lefever S, Van Den Bossche D, Van Moerkercke W, et al. Ruminococcus gnavus bacteremia, an uncommon presentation of a common member of the human gut microbiota: case report and literature review. Acta Clin Belg 2019;74:435-8. [Crossref] [PubMed]
- Titécat M, Wallet F, Vieillard MH, et al. Ruminococcus gnavus: an unusual pathogen in septic arthritis. Anaerobe 2014;30:159-60. [Crossref] [PubMed]
- Fernández-Caso B, Domingo García D, Domingo LC, et al. Ruminococcus gnavus infection of a metal-on-metal hip arthroplasty resembling a pseudo-tumour in a 72 year-old woman with no intestinal symptoms. Enferm Infecc Microbiol Clin 2017;35:542-3. [Crossref] [PubMed]
- Roux AL, El Sayed F, Duffiet P, et al. Ruminococcus gnavus total hip arthroplasty infection in a 62-year-old man with ulcerative colitis. J Clin Microbiol 2015;53:1428-30. [Crossref] [PubMed]
- Arnáez Solís R, Martín Salas C, Acha Arrieta MV. Prosthetic joint infection by Ruminococcus gnavus in a patient without associated digestive disease. Med Clin (Barc) 2017;148:e7-8. [Crossref] [PubMed]



