
Thoracic spondylodiscitis secondary to Klebsiella oxytoca urosepsis—a case report
Introduction
Spondylodiscitis is an infectious pathology of the spine that is challenging to diagnose in its early stages and can lead to significant morbidity (1). Up to five percent of cases of osteomyelitis involve the vertebral column. Diagnostic delays occur frequently and the time from symptom onset to diagnosis is often in the order of weeks to months (2). Spondylodiscitis occurs when there is inoculation of micro-organisms into the subchondral vertebral body endplates or intervertebral discs, which spread to contiguous vertebral bodies. Frequently, the paravertebral musculature may become involved, with phlegmon then abscess formation. Infection may extend into the spinal extradural space, and compress neural elements causing radiculopathy and myelopathy. Spondylodiscitis is characterised by non-specific axial back pain that worsens in subacute fashion before a deterioration in symptoms prompts medical intervention. It may lead to marked spinal deformity, spinal cord and/or cauda equina compression, sepsis, and death. Spondylodiscitis has myriad aetiologies. It can occur secondary to spinal trauma, surgery (especially following instrumentation), local spread from adjacent soft tissue infection, and haematogenous seeding from a distant site (1,2). Spondylodiscitis may precede or follow a bacteraemia, and is most often a single pathogen infection (1). The most implicated bacterium and infectious agent overall is Staphylococcus aureus, which is usually disseminated from a distant origin, often cutaneous (2). Escherichia coli is the next most common organism, and seeds following urinary tract or enteric infections. When infection follows spinal surgery, the organisms responsible are commonly skin commensals, such as Cutibacterium acnes and coagulase-negative Staphylococcus spp. Inoculation of these organisms occurs due to a failure of asepsis intraoperatively. Pseudomonas spp. are identified occasionally and occur in patients with a history of illicit intravenous (IV) drug use, or exposure to unchlorinated pool water (2). Spondylodiscitis secondary to Mycobacterium tuberculosis, Brucella spp., and fungi, is more often seen in people who reside in endemic regions, and these are rarer pathogens overall (1,2).
Factors that predispose to a greater risk of spondylodiscitis include advanced age, immunosuppression, poor glycaemic control, concomitant malignancy, malnutrition, and illicit IV drug use. The incidence worldwide has been reported at rates of up to 22 persons per 100,000, with a slight male preponderance. Morbidity and mortality vary with severity of disease at diagnosis, micro-organism, and various patient factors (1-3).
Klebsiella spp. are bacteria of the Enterobacterales and Enterobacteriaceae, order and family, respectively. They are normal flora of the gastrointestinal tract and can be found in water and soil (4). Klebsiella oxytoca is a non-motile Gram stain negative bacillus that is distinguished from Klebsiella pneumoniae by the absence of tryptophanase and thus, its inability to convert tryptophan to indole (5,6). Klebsiella spp. are important infectious pathogens, clades of which possess antimicrobial resistance. They have been shown to share core and accessory, antimicrobial resistance and virulence factor genes through recombination (5). Klebsiella spp. are known biliary, integumentary, urinary, and pulmonary pathogens, and can also infect intravascular devices. There are few reports of K. oxytoca as the causative micro-organism in spondylodiscitis in the medical literature (only five to our knowledge) (6-8), while there is a comparably greater number of cases attributed to K. pneumoniae (3,8,9). In the present study, we report a rare case of an elderly gentleman who presented with subacute back pain secondary to urosepsis-associated K. oxytoca spondylodiscitis, with neurological compromise, treated with posterior surgical decompression and debridement, screw-rod fixation, and long-term antibiotic therapy. We present the following article in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-124/rc).
Case presentation
A 79-year-old Slavic male presented to his local medical officer with dysuria. He had a body mass index of 34 and a Charlson comorbidity index score of three. He was a retiree, independent with his activities of daily living, and resided at home. His past medical history was significant for controlled essential hypertension, osteopenia, previous urinary tract infection (UTI) associated with nephrolithiasis requiring ureteric stenting, and symptomatic prostatic enlargement, for which he was awaiting transurethral resection. Urine culture identified K. oxytoca, which was susceptible to cephazolin. He was prescribed a 7-day course of cephalexin.
One month later, he was admitted to hospital, febrile with urosepsis, with a complaint of worsening mid-thoracic back pain. Computed tomography (CT) of the spine was unremarkable. K. oxytoca was again isolated from blood and urine cultures, and there was new cephazolin resistance evident on susceptibility testing. He was treated with 3 days of IV amoxycillin/clavulanate, followed by 3 days of IV ceftriaxone, and a further 7 days of oral cephalexin on discharge.
He represented to hospital 1 month later. Repeat CT demonstrated interval development of marked thoracic kyphotic deformity with canal stenosis, secondary to an ostensible three-column fracture of T5, with a 90% loss of vertebral body height. He was slightly confused, denied any antecedent trauma and was otherwise neurologically intact. There was evidence of an underlying inflammatory process on routine blood testing [C-reactive protein (CRP) was elevated to 118.4 mg/L], but there was no associated leukocytosis. He had a low-grade fever on representation but serial blood cultures failed to grow any further micro-organisms. Magnetic resonance imaging (MRI) of the whole spine was performed. This demonstrated pathological erosion and fracture of the T5 and T6 vertebrae, with surrounding ectopic T2-hyperintensity and diffuse intravertebral and paravertebral gadolinium enhancement. There was an extradural phlegmon present, and myelomalacia was evident. This radiology is depicted in Figure 1. The clinical context and radiological constellation were suggestive of a diagnosis of spondylodiscitis with associated myelopathy.
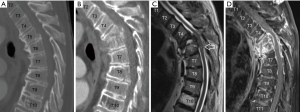
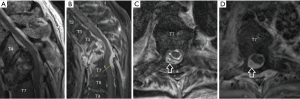
Our patient was initially managed conservatively. His intact neurology, advanced age, and frail and delirious state on admission were considered; surgery was thought to be high risk. He was fitted with a thoracolumbosacral orthosis and permitted to mobilise. Needle biopsy of the T5 lesion was performed under fluoroscopic guidance, and culture again yielded K. oxytoca. CT IV pyelography was performed. There was no radiological evidence of acute prostatitis or pyelonephritis, although the prostate was enlarged with coarse calcifications. Transthoracic echocardiography was unremarkable. IV ceftriaxone was commenced, and the antibiogram (see Table 1) demonstrated susceptibility with a minimum inhibitory concentration of less than 1 mg/L. Nine days after admission, our patient began to retain urine and was catheterised. This event was thought to be related to his known prostate issues, and there was no other accompanying neurological deterioration. Three weeks following admission, our patient developed acute lower limb weakness and paraesthesia, which was more pronounced on the right. Repeat MRI and CT demonstrated interval deterioration of the existing spinal deformity with worsening cord compression, kyphosis and right-sided posterior spinal extradural abscess. This radiology is depicted in Figure 2. Our patient underwent emergent posterior thoracic decompression and surgical debridement. Laminectomies were performed from T5-7. The abscess was extricated with blunt dissection. Pedicle screws were inserted bilaterally at T3, T4, T7, and T8, and fixed with curved rods posteriorly. His post-operative radiology is depicted in Figure 3. Anterior decompression and large-scale osteotomy were deemed inappropriate due to our patient’s performance status, age and frailty.
Table 1
| Antibiotic | Disk zone (mm) | Disk interpretation | VITEK2 mean inhibitory concentration (mg/L) | VITEK2 interpretation |
|---|---|---|---|---|
| Amikacin | 22 | Susceptible | ≤2 | Susceptible |
| Amoxycillin/clavulanate | 25 | Susceptible | 4 | Susceptible |
| Ampicillin | ≤6 | Resistant | ≥32 | Resistant |
| Cefepime | 34 | Susceptible | ≤1 | Susceptible |
| Ceftazidime | Not tested | ≤1 | Susceptible | |
| Ceftriaxone | 31 | Susceptible | ≤1 | Susceptible |
| Cephazolin | 17 | Resistant | ≥64 | Resistant |
| Ciprofloxacin | 32 | Susceptible | ≤0.25 | Susceptible |
| Gentamicin | 21 | Susceptible | ≤1 | Susceptible |
| Meropenem | 34 | Susceptible | ≤0.25 | Susceptible |
| Norfloxacin | Not tested | ≤0.5 | Susceptible | |
| Tobramycin | Not tested | ≤1 | Susceptible | |
| Trimethoprim/sulfamethoxazole | 30 | Susceptible | ≤20 | Susceptible |
Table showing the antibiotic resistances and susceptibilities of our patient’s strain of K. oxytoca as determined by EUCAST standardised disk diffusion testing, as well as those determined using the bioMérieux VITEK 2 mass spectrometry automated micro-broth dilution machine. Testing was performed on the strain isolated from blood culture during the first hospital admission. The interpretation of the results was performed in accordance with the EUCAST guidelines. EUCAST, The European committee on antimicrobial susceptibility testing.
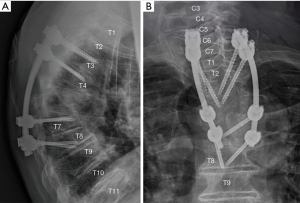
Post-operatively, the motor deficits improved, although there were residual sensory disturbances. The patient was treated with ceftriaxone 2 g daily IV for 1 further week, then his therapy was de-escalated to ciprofloxacin 750 mg twice daily orally, aiming for a total duration of 12 weeks following surgery. An extended period of therapy was chosen due to concerns regarding insertion of prosthetic material into a site of active infection. Ciprofloxacin was selected due to its high bioavailability and anti-biofilm activity. Our patient’s CRP gradually waned to 12 mg/L at the time of discharge (6 weeks post-operatively) and this had normalised by 12 weeks (4 mg/L). He eventually returned to his own home after a period of inpatient rehabilitation. He had persisting right-sided lower limb weakness, and bilateral sensory deficits including proprioceptive loss. His urinary function did not recover, and he required long-term suprapubic catheterisation.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Klebsiella spp. are opportunistic pathogens, responsible for both community-acquired and nosocomial infections. The species most implicated is K. pneumoniae and this is the second most preponderous hospital-acquired infection after E. coli (4). K. oxytoca is comparably less common and has a similar antibiotic resistance profile to K. pneumoniae. Historically, K. oxytoca was thought to be more prevalent in the paediatric intensive care unit setting, however there is a rising incidence of infection in critically ill adult patients (4). Nosocomial isolates have demonstrated multi-resistance to antibiotics. Genes encoding extended-spectrum and AmpC-type β-lactamases, carbapenemases and other enzymes, confer resistance to those that have a β-lactam ring moiety, such as cephalosporins and penicillins (4). These genes are chromosome and plasmid-borne, and their expression can be constitutive or induced (4,5,10,11). The strain isolated in our patient was not subject to further genotypic testing after identification. Moradigaravand et al. [2017] (5) analysed the drug resistance profiles of isolates of K. oxytoca from blood cultures of hospitalised patients in the United Kingdom and Ireland. A wide spectrum of drug resistance was detected, reflecting plasmid exchange of antimicrobial resistance genes between K. oxytoca and other nosocomial bacteria. Singh et al. [2016] (4) demonstrated greater rates of resistance amongst strains of K. oxytoca to β-lactams, aminoglycosides, and fluoroquinolones, as compared with strains of K. pneumoniae isolated in an Indian tertiary hospital.
Our patient incurred K. oxytoca urosepsis in the context of significant urological pathology. His presentation was clinically indistinguishable from other bacterial spondylodiscitis. K. oxytoca often possess intrinsic resistance to penicillin via chromosomally encoded SHV-1 β-lactamase (12,13). Such strains may also express plasmid-encoded SHV-1, TEM-1, and TEM-2 β-lactamases. Inducible or inherent, hyper-expression or co-expression of these enzymes can potentiate a broader antimicrobial resistance profile, including cephalosporins (14,15), and cephalexin resistance has been documented (16). We infer that our patient was afflicted by such a strain. Our patient’s initial urine isolate of K. oxytoca was susceptible to cephazolin, then during hospitalisation, repeat culture and antibiogram identified cephazolin resistance. This may have been induced or inadvertently selected, by cephalexin therapy. We hypothesise that our patient’s complex urology resulted in incomplete microbial clearance following treatment, with ongoing colonisation, followed by a recurrent infection and bacteraemia, with resultant vertebral seeding. There was some contextual evidence of chronic prostatic inflammation clinically, and radiologically as depicted in Figure 4. Furthermore, cephalexin penetration of the prostate is poor, and the prostate is a potential reservoir for a bacterial niche to evade antibiosis (17). Prostatic obstruction of the urinary outflow tract may have contributed to inadequate urinary clearance of pathogens also. De-escalation to cephalexin to which this strain was resistant, compounded this unfortunate series of events.
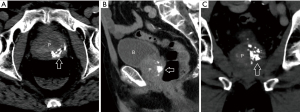
Batson’s venous plexus is a likely pathway of dissemination to the vertebral column in infectious metastases of urological origin. It is a valveless network that communicates with the major venous plexuses of the pelvic organs and the internal vertebral venous plexus (18). This assertion is supported by the frequent observation of infection of the subchondral vertebral endplates at contiguous spinal levels, suggesting a common vascular route of pathogens (7,19,20).
K. oxytoca is an obscure pathogen overall, and we suspect that its role in spinal infection is under-reported. Sabio et al. [2002] (6) reported a case of conservatively managed K. oxytoca spondylodiscitis of the thoracolumbar junction, in a 51-year-old male with a history of illicit IV drug use, and an alleged association with skin ulcers. Burkhardt et al. [2019] (8) reported a series of cases of infectious cervical spondylodiscitis treated with anterior corpectomy and arthrodesis. This included a singular episode implicating K. oxytoca, although this was not specifically characterised further. Agrawal et al. [2009] (7) reported a case of cervical polymicrobial spondylodiscitis due to dissemination of Enterobacter cloacae and K. oxytoca, following transrectal ultrasound-guided prostatic biopsy, also managed conservatively. Park et al. [2016] (21) retrospectively reviewed 345 cases of spondylodiscitis, two of which were due to K. oxytoca.
The primary indication for surgical fixation in spondylodiscitis is spinal instability. Lang et al. [2021] (22) and Dietze et al. [1997] (23) provided criteria for the assessment of potential spinal instability based on the latter authors’ experience with 20 such patients, and pre-existing definitions of instability including Denis’ three column concept. The presence of any one of the following criteria is potentially indicative of spinal instability. These are: (I) the presence of segmental kyphosis greater than 15–20°; (II) a loss of vertebral height at a single level of 50% or more; and/or (III) translation of a vertebral segment 5 mm or more anteriorly or posteriorly on dynamic radiographs. In the case of our patient, both (I) and (II) criteria were present on repeat CT imaging when he first represented. The decision to manage him conservatively initially was based on his frailty, but then a deterioration in his neurology eventually necessitated surgical intervention.
The gold standard of diagnostic imaging of spondylodiscitis is MRI (24). Microbiological identification of causative organisms, to direct antibiotic therapy is imperative. Blood cultures should be drawn, and these should be obtained from multiple venepuncture sites to prevent confusion associated with false positives due to contaminant organisms (2). Percutaneous biopsy under imaging guidance and subsequent specimen culture may be performed to elucidate the causative microbiology. Occasionally, in the absence of positive results, contextual medical history, such as past infection with a known urinary pathogen as in our patient, can be used to guide antibiotic choice.
Conclusions
Spondylodiscitis is an insidious pathology with potential for severe morbidity and mortality. Clinicians should have a low threshold of suspicion for spondylodiscitis in patients with subacute worsening back pain and risk factors. Inadequate treatment of UTI can have devastating consequences. Patients with complex urology should have close follow-up to ensure adequate urinary clearance of pathogenic organisms. Appropriate choice of antibiotic therapy is imperative. Klebsiella spp. are an important cause of drug-resistant UTI and possess intrinsic antibiotic resistance mechanisms. K. oxytoca is a rare and probably under-reported cause of spondylodiscitis. Best outcomes require mobilisation of multidisciplinary expertise.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-124/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-124/coif). RP serves as an unpaid editorial board member of Journal of Spine Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of it are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Graeber A, Cecava ND. Vertebral osteomyelitis. StatPearls. Treasure Island (FL): StatPearls Publishing, 2022.
- Chenoweth CE, Bassin BS, Mack MR, et al. Vertebral osteomyelitis, discitis, and spinal epidural abscess in adults. Ann Arbor (MI): Michigan Medicine University of Michigan, 2018.
- Schattner A, Drahy Y. Nosocomial vertebral osteomyelitis. Am J Med 2017;130:e309-10. [Crossref] [PubMed]
- Singh L, Cariappa MP, Kaur M. Klebsiella oxytoca: an emerging pathogen? Med J Armed Forces India 2016;72:S59-61. [Crossref] [PubMed]
- Moradigaravand D, Martin V, Peacock SJ, et al. Population structure of multidrug resistant Klebsiella oxytoca within hospitals across the UK and Ireland identifies sharing of virulence and resistance genes with K. pneumoniae. Genome Biol Evol 2017;9:574-87. [Crossref] [PubMed]
- Sabio JM, López-Gómez M, Jiménez-Alonso J. Spontaneous spondylodiscitis caused by Klebsiella oxytoca. Ann Rheum Dis 2002;61:758-9. [Crossref] [PubMed]
- Agrawal S, Patil K, Dunsmuir WD. A pain in the neck—an unexpected complication of transrectal ultrasound and biopsy. Br J Radiol 2009;82:e92-4. [Crossref] [PubMed]
- Burkhardt BW, Müller SJ, Wagner AC, et al. Anterior cervical spine surgery for the treatment of subaxial cervical spondylodiscitis: a report of 30 consecutive patients. Neurosurg Focus 2019;46:E6. [Crossref] [PubMed]
- Thakur NA, Schiller JR, Fischer SA, et al. Klebsiella pneumoniae-associated vertebral osteomyelitis after laparoscopic cholecystectomy. Hosp Pract (1995) 2010;38:75-8. [Crossref] [PubMed]
- Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009;22:161-82. Table of Contents. [Crossref] [PubMed]
- Meini S, Tascini C, Cei M, et al. AmpC β-lactamase-producing enterobacterales: what a clinician should know. Infection 2019;47:363-75. [Crossref] [PubMed]
- Babini GS, Livermore DM. Are SHV beta-lactamases universal in klebsiella pneumoniae? Antimicrob Agents Chemother 2000;44:2230. [Crossref] [PubMed]
- Chaves J, Ladona MG, Segura C, et al. SHV-1 beta-lactamase is mainly a chromosomally encoded species-specific enzyme in Klebsiella pneumoniae. Antimicrob Agents Chemother 2001;45:2856-61. [Crossref] [PubMed]
- Miró E, del Cuerpo M, Navarro F, et al. Emergence of clinical Escherichia coli isolates with decreased susceptibility to ceftazidime and synergic effect with co-amoxiclav due to SHV-1 hyperproduction. J Antimicrob Chemother 1998;42:535-8. [Crossref] [PubMed]
- Rice LB, Carias LL, Hujer AM, et al. High-level expression of chromosomally encoded SHV-1 beta-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob Agents Chemother 2000;44:362-7. [Crossref] [PubMed]
- Nguyen HM, Graber CJ. A critical review of cephalexin and cefadroxil for the treatment of acute uncomplicated lower urinary tract infection in the era of “bad bugs, few drugs”. Int J Antimicrob Agents 2020;56:106085. [Crossref] [PubMed]
- Lipsky BA, Byren I, Hoey CT. Treatment of bacterial prostatitis. Clin Infect Dis 2010;50:1641-52. [Crossref] [PubMed]
- Tsutsumi R, Saito M, Yoshizawa T. Group B streptococcus meningitis and infection surrounding the spinal canal caused by bacterial transmission from rectal ulcer via Batson’s plexus. Rinsho Shinkeigaku 2011;51:493-8. [Crossref] [PubMed]
- Kumar R. Spinal tuberculosis: with reference to the children of northern India. Childs Nerv Syst 2005;21:19-26. [Crossref] [PubMed]
- Ziu E, Viswanathan VK, Mesfin FB. Spinal metastasis. StatPearls. Treasure Island (FL): StatPearls Publishing, 2022.
- Park KH, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016;62:1262-9. [Crossref] [PubMed]
- Lang S, Rupp M, Hanses F, et al. Infections of the spine: pyogenic spondylodiscitis and implant-associated vertebral osteomyelitis. Unfallchirurg 2021;124:489-504. [Crossref] [PubMed]
- Dietze DD Jr, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg 1997;86:981-9. [Crossref] [PubMed]
- Ahn KS, Kang CH, Hong SJ, et al. The correlation between follow-up MRI findings and laboratory results in pyogenic spondylodiscitis. BMC Musculoskelet Disord 2020;21:428. [Crossref] [PubMed]


