
Tracking the disease progression of lumbar spinal stenosis using objective gait metrics: a case report
Introduction
Lumbar spinal stenosis (LSS) is a common disease and has a prevalence between 11% and 39% in the general population based on clinical diagnoses (1). LSS is often a degenerative disease caused by a narrowing of the spaces within the intraspinal lumbar canal, typically due to the intrusion of adjacent structures such as a hypertrophied ligamentum flavum or the protrusion of a degenerating intervertebral disc (2). These processes may cause irritation or ischaemia of the entrapped nerve roots, resulting in neurogenic claudication—a clinical syndrome of back or leg pain, weakness, and paraesthesia that is exacerbated by walking and relieved by lumbar flexion (3). As the stenosis worsens it affects the patient’s capacity to walk long distances and may result in changes to walking patterns where patients adjust the position of their pelvis, torso, and legs to alleviate pain, or compensate for weakness. These changes often come at the cost of deteriorating walking patterns, and patients may need increasing levels of walking assistance (such as a walking stick) as the disease progresses (2,3).
It is well established that LSS causes a deterioration of objective and quantifiable gait and walking metrics (4). Studies have reported that, compared to healthy controls, LSS patients have decreased walking speed, cadence, and step length, and increased step time, double support time, stance phase time, swing phase time, and gait variability (5-8). Alongside this, gait patterns can provide insight into LSS disease severity, although routine evaluation of gait metrics is not a standardized measure of care. LSS patients with higher levels of pain and functional disability (as measured using patient-reported outcome measures) have worse spatiotemporal gait metrics and patients with higher radiological grades of stenosis demonstrate greater pelvic rigidity (9,10).
In this case report, we demonstrate objective evidence of gait deterioration in an elderly patient with LSS. We hypothesize that routine remote observation of gait measures can have significant clinical utility in not just detection of symptom progression but also as an adjunct for clinical decision-making. We present the following case in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-101/rc).
Case presentation
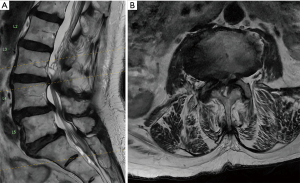
An 85-year-old woman presented to the NeuroSpine Clinic (Randwick, Australia) with neurogenic claudication over a period of two years. Despite being elderly, her medical background did not report any other comorbidities and she was otherwise from home with an independent baseline. Qualitative gait observations revealed a slouching posture with symptoms worsening when standing upright for several minutes. Upper motor neuron signs were not reported. Her magnetic resonance imaging (MRI) revealed severe stenosis equating to grade D compression, confirming her diagnosis of LSS (Figure 1).
The patient first presented during October 2019. For the subsequent two years, her daily step count was recorded using her smartphone (iPhone) which she kept on her person at nearly all times. Using a stopwatch and surveyor’s wheel, walking speed and step length were also independently assessed across three timepoints (October 2019, March 2021, July 2021) with the patient walking a self-selected distance (maximum 120 m) at a self-selected pace. This data is summarized in Table 1. She did not develop any other medical comorbidities during this time and her walking deterioration was likely secondary to her worsening lumbar stenosis.
Table 1
| Date | Steps per day | Gait velocity (m/s) | Stride length (m) |
|---|---|---|---|
| October 2019 | 3,136 | 1.03 | 0.49 |
| November 2019 | 3,093 | − | − |
| December 2019 | 2,929 | − | − |
| January 2020 | 3,097 | − | − |
| February 2020 | 2,838 | − | − |
| March 2020 | 2,610 | − | − |
| April 2020 | 2,772 | − | − |
| May 2020 | 2,139 | − | − |
| June 2020 | 2,444 | − | − |
| July 2020 | 2,272 | − | − |
| August 2020 | 1,991 | − | − |
| September 2020 | 1,932 | − | − |
| October 2020 | 1,878 | − | − |
| November 2020 | 1,529 | − | − |
| December 2020 | 1,493 | − | − |
| January 2021 | 1,265 | − | − |
| February 2021 | 1,277 | − | − |
| March 2021 | 854 | 0.97 | 0.41 |
| April 2021 | 858 | − | − |
| May 2021 | 639 | − | − |
| June 2021 | 612 | − | − |
| July 2021 | 334 | 0.49 | 0.37 |
| August 2021 | 301 | − | − |
As shown in Figure 2, her initial walking metrics (walking speed =1.03 m/s, step length =0.49 m, and daily step count =3,136) were all above age- and sex-matched normative values (walking speed =0.94 m/s, step length =0.55 m, and approximate daily step count =1,500), suggestive of only mild functional disability (11-13). After neurosurgical evaluation, conservative therapies including physiotherapy, analgesics, and steroid injections were recommended. No walking aid was required.
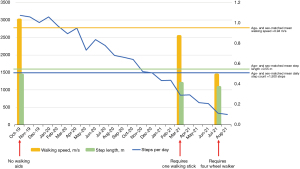
When assessed again in March 2021, the patient’s walking metrics had significantly deteriorated (walking speed =0.97 m/s, step length =0.41 m, and daily step count =854) and she required a walking stick for all mobility. This pattern continued and in July 2021, her metrics were all below normative values (walking speed =0.49 m/s, step length =0.37 m, and daily step count =334). At this time, she required a four-wheel walker and could only mobilize for a few metres before she needed to rest. Physical examination confirmed bilateral lower limb weakness and paraesthesia upon exertion. As her clinical symptoms had deteriorated and were now significantly affecting her quality of life, surgical management was recommended after neurosurgical evaluation and a shared decision-making process with the patient and her family.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This study was approved by the South-Eastern Local Health District Human Research Ethics Committee, with the reference number 17/184. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
LSS is a common degenerative disease of the lumbar spine which can affect a person’s walking patterns. Objectively monitoring walking metrics in these people may provide insight into fluctuations in their health status (4,14). To our knowledge, this is the first recorded case actively tracking the decline of a patient with LSS by objectively measuring their walking patterns for such a prolonged duration. By doing so, we have demonstrated correlation between deteriorating walking metrics and an increased need for walking assistance.
Wearable devices (such as smartphones, goniometers, activity trackers, etc.) can be used to objectively measure a person’s walking metrics. These devices are already widely used on a consumer basis—smartphones, for instance, have an estimated 5 billion users worldwide as of 2019 (15). This represents significant public trust, and clinicians across different specialties (but particularly those involved with gait-altering diseases) may use smartphone-captured walking metrics (typically daily step counts) to gauge their patient’s walking ability amongst other functional measures. Although not demonstrated in this report (wherein we used a stopwatch and surveyor’s wheel for further additional measurements at discrete timepoints as part of our routine clinic assessment), current wearable devices can also capture core spatiotemporal gait metrics such as walking speed and step time, with some being able to measure their respective derivations of asymmetry and variability (16). A systematic review by Stienen et al. (17) in 2019 revealed that other forms of objective outcome measurement primarily include clinician-observed tests such as the timed up and go test, the motorized treadmill test, and the self-paced walking test. These appeared in 9.8–31.7% of papers incorporating the objective outcome analysis of spine patients. However, the frequency of these assessments is limited to in-person presentations and cannot match the day-to-day monitoring of walking patterns made possible using wearable devices.
Additional benefits of wearable devices include their potential for the remote monitoring of patients while in their everyday environments, and the continuous collection of data for as long as the device is worn (18). Other forms of walking analysis include three-dimensional motion capture systems which may be combined with force plates that measure ground-reaction forces. Although the gold-standard in gait analysis, these methods are expensive, time-consuming and require expert operation and equipment at discrete time points, thereby having inferior clinical utility to wearable devices (18).
To facilitate ambulation and prevent falls-related accidents, walking assistance (such as a walking stick, or four-wheel walker) is needed when a person’s walking patterns deteriorate. Currently, requirements for walking assistance are based on a process of clinical evaluation, often supplemented by allied health assessments that may be difficult to arrange or time-consuming. Our patient’s walking assistance requirements aligned with her deteriorating walking metrics, which dipped far below their original values at first presentation (original: walking speed =1.03 m/s, step length =0.49 m, daily step count =3,136; latest: walking speed =0.49 m/s, step length =0.37 m, and daily step count =334) as shown in Figure 2. In conjunction with clinical examination, this was reflective of the progressive LSS disease process, whereby conservative management strategies were not adequate in recovery or prevention of further deterioration. It is also important to recognise that across this period the patient did not have any falls or other confounders for deterioration.
In this case we have demonstrated the potential for walking metrics to stratify impairment based on severity and based on these findings make recommendations for the degree of walking aids required. However, the present report is limited by sample size, and future studies are required to consolidate these findings before they can be tangibly translated into clinical contexts. Nonetheless, our previous case series in patients undergoing spine surgery also demonstrated utility in basic walking metrics as outcome measures with subjects demonstrating a statistically significant improvement in daily step count at 3-month follow-up (58.2% increase, P=0.008) (19).
In this report, we have been able to demonstrate a trajectory pathway of deterioration in a subject with LSS. With more advanced measures being employed in even affordable commercial devices, the understanding of gait patterns in pathology remains an evolving field, and recognising pathology based on gait pattern has been widely established across clinical examination. Naturally, this represents a potential target for novel devices, and we speculate that with time and the shift during the pandemic into methods of telehealth and remote assessment, such devices will become integrated and form an irreplaceable component of clinical assessment.
Conclusions
LSS disease progression can be remotely visualised using simple phone derived walking metrics. The extent of walking deterioration may be used to inform clinical decision-making regarding appropriate walking assistance and assessment of treatment effectiveness. This objective concept, with the advent of evolving device capabilities may bring about more refined decision making and personalised remote healthcare soon in both LSS and other conditions impacting gait and general health overall.
Acknowledgments
The NeuroSpine Clinic aided with recruitment of our patient.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Spine Surgery for the series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-101/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-21-101/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-101/coif). The series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” was commissioned by the editorial office without any funding or sponsorship. RJM served as the unpaid Guest Editor of the series and serves as the Editor-in-Chief of Journal of Spine Surgery. RDF and PN served as the unpaid Guest Editors of the series and serve as unpaid Assistant Managing Editors of Journal of Spine Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This study was approved by the South-Eastern Local Health District Human Research Ethics Committee, with the reference number 17/184. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jensen RK, Jensen TS, Koes B, et al. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. Eur Spine J 2020;29:2143-63. [Crossref] [PubMed]
- Lee BH, Moon SH, Suk KS, et al. Lumbar Spinal Stenosis: Pathophysiology and Treatment Principle: A Narrative Review. Asian Spine J 2020;14:682-93. [Crossref] [PubMed]
- Siebert E, Prüss H, Klingebiel R, et al. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol 2009;5:392-403. [Crossref] [PubMed]
- Chakravorty A, Mobbs RJ, Anderson DB, et al. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: systematic review. BMC Musculoskelet Disord 2019;20:288. [Crossref] [PubMed]
- Li YG, Li LP, Li ZJ, et al. Gait analysis in the elderly patients with lumbar spinal stenosis. Int Orthop 2021;45:673-9. [Crossref] [PubMed]
- Loske S, Nüesch C, Byrnes KS, et al. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. Spine J 2018;18:2195-204. [Crossref] [PubMed]
- Perring J, Mobbs R, Betteridge C. Analysis of Patterns of Gait Deterioration in Patients with Lumbar Spinal Stenosis. World Neurosurg 2020;141:e55-9. [Crossref] [PubMed]
- Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas 2009;30:1171-86. [Crossref] [PubMed]
- Bumann H, Nüesch C, Loske S, et al. Severity of degenerative lumbar spinal stenosis affects pelvic rigidity during walking. Spine J 2020;20:112-20. [Crossref] [PubMed]
- Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919-24. [Crossref] [PubMed]
- Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy 2011;97:182-9. [Crossref] [PubMed]
- Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011;34:111-8. [Crossref] [PubMed]
- Tudor-Locke C, Schuna JM Jr, Barreira TV, et al. Normative steps/day values for older adults: NHANES 2005-2006. J Gerontol A Biol Sci Med Sci 2013;68:1426-32. [Crossref] [PubMed]
- Mobbs RJ, Mobbs RR, Choy WJ. Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: the Gait Posture index (GPi). J Spine Surg 2019;5:300-9. [Crossref] [PubMed]
- Abdulla MA, Esmaeel AM. Providing Information through Smart Platforms: An Applied Study on Academic Libraries in Saudi Universities. Journal of Education Society and Behavioural Science 2019;30:1-24. [Crossref]
- Patel M, Pavic A, Goodwin VA. Wearable inertial sensors to measure gait and posture characteristic differences in older adult fallers and non-fallers: A scoping review. Gait Posture 2020;76:110-21. [Crossref] [PubMed]
- Stienen MN, Ho AL, Staartjes VE, et al. Objective measures of functional impairment for degenerative diseases of the lumbar spine: a systematic review of the literature. Spine J 2019;19:1276-93. [Crossref] [PubMed]
- Benson LC, Clermont CA, Bošnjak E, et al. The use of wearable devices for walking and running gait analysis outside of the lab: A systematic review. Gait Posture 2018;63:124-38. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Maharaj M, et al. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Global Spine J 2016;6:459-64. [Crossref] [PubMed]


