
Analysing gait patterns in degenerative lumbar spine diseases: a literature review
Introduction
Low back pain (LBP) is a common health problem that accounts for a substantial clinical and socioeconomic global health burden incurring a total measured burden of approximately 83 million disability-adjusted life years (1). Most cases of LBP (other than non-specific causes) involve well-defined pathoanatomical causes (pain generators) and are typically associated with degenerative diseases of the lumbar spine (2,3).
Degenerative lumbar spine diseases affect 266 million people worldwide and includes diagnoses such as lumbar spinal stenosis (LSS), lumbar disc herniation (LDH) and mechanical-type (discogenic or facetogenic) LBP (4). These diseases can present with a range of symptoms such as sciatica, neurogenic intermittent claudication and mechanical-type onset of LBP respectively (4). Although varying in symptoms and severity, degenerative lumbar diseases are theorised to be associated with biomechanical impairments of spinal muscles resulting in energy-inefficient gait patterns (5) and therefore a deterioration to walking quality and capacity (6,7).
Gait is a clinically important biomarker for the identification and evaluation of disease-states (8). Performance-oriented functional tests of gait such as the 10-meter walk (10MW) test, 6-minute walking (6MWT) test, or Timed Up and Go (TUG) typically focus on a single quantitative parameter of gait (6,7), overlooking other important aspects such as quality of gait (9). They also do not reveal specifically which aspects of gait differ from healthy gait. Previously, several quantitative gait analysis studies have investigated the walking patterns of lumbar degenerative diseases, quantifying the spatial and temporal aspects of gait deterioration (10-12).
To the best of the authors’ knowledge however, a review of literature has not been undertaken to compare the gait patterns in these lumbar degenerative diseases including LSS, LDH and chronic mechanical-type LBP. Non-specific causes of LBP were not considered in the present review with LBP referring to chronic mechanical-type onset and presence of patho-anatomical (facetogenic or discogenic) pain generators. Analysing gait patterns in lumbar spine diseases, may pave the way for the creation of a disease-specific gait profiles to aid clinical identification of disease-states. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-21-91/rc).
Methods
Spatial and temporal gait parameters
During a bout of walking, two successive steps such that a foot returns to its initial position is considered a ‘stride’ or a ‘gait cycle’ (Figure 1). Within a gait cycle the two distinct phases of a foot can be considered: stance (foot is in contact with ground) and swing (foot is lifted and moved forwards) (13). Moreover, the time that both feet touch the ground (double support time) and only one foot touches the ground (single support time) can also be analysed. These gait phases can be analysed as a proportion of gait cycle and compared to normative values: stance (60%), swing (40%) and double support time ratios (20%).
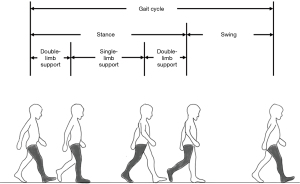
The walking bout itself may quantitatively analysed (Table 1) considering spatial (step and stride length) and temporal parameters (step and stride time) and these may all be computed with minimal equipment. Composite ‘spatiotemporal’ measures of gait can be derived from these variables: walking speed and cadence. More complex ‘derived’ metrics include gait variability (step-to-step variation) and asymmetry (average difference between left and right foot). Step (rather than stride) measurements reflect gait variability more reliably (14).
Table 1
| Gait variable | Definition | Units | Type |
|---|---|---|---|
| Step length | Average distance between two consecutive contacts of any foot with the ground | Metres (m) | Spatial |
| Stride length | Average distance between two consecutive contacts of the same foot with the ground | Metres (m) | Spatial |
| Step time | Average time between two consecutive contacts of any foot with the ground | Seconds (s) | Temporal |
| Stride time | Average time between two consecutive contacts of the same foot with the ground | Seconds (s) | Temporal |
| Walking speed (or gait velocity) | Average distance travelled per second | Metres/second (m/s) | Spatiotemporal |
| Cadence | Average rate (or frequency) of steps | Steps/minute | Spatiotemporal |
| Step time variability | Step-to-step variability of step time | Standard deviation (SD) coefficient of variance (cov = SD/mean) | Gait variability |
| Step length variability | Step-to-step variability of step length | Standard deviation (SD) coefficient of variance (cov = SD/mean) | Gait variability |
| Walking speed (or gait velocity) variability | Step-to-step variability of walking speed | Coefficient of variance (cov = SD/mean) | Gait variability |
| Step time asymmetry | Average difference in time taken for successive steps on left and right foot | Seconds (s) | Gait asymmetry |
| Step length asymmetry | Average difference in length for successive steps on left and right foot | Metres (m) | Gait asymmetry |
Objectives
A search of the literature was conducted to determine the changes in spatial and temporal gait metrics involved with each type of degenerative lumbar spine disease: LSS, LDH and chronic LBP.
Literature search
A search strategy was created to identify relevant studies (Appendix 1). Medline, Embase and PubMed databases were searched from their date of inception to April 18th, 2021. Relevant articles were screened (Appendix 2) according to:
Eligibility criteria
Inclusion criteria
- Original articles investigating spatiotemporal gait metrics;
- Articles involving at least one (or more) lumbar spine pathology groups (LSS, LDH, LBP) and/or healthy control groups;
- Articles written in English;
- Human studies published between 1980–April 2021.
Exclusion criteria
- Studies investigating other variables of gait e.g., range-of-motion, tri-dimensional forces, posture;
- Studies without comparative healthy control participants;
- Studies investigating normal human gait activities (e.g., running, walking);
- Studies investigating gait metrics associated with interventions (surgical or pharmacological);
- Reviews, Conference Abstracts, Books.
Data collection
Differences in gait parameters were abstracted from included studies as a percentage difference compared to healthy controls. These variables include: (I) Gait Velocity; (II), Cadence; (III) Gait Lengths (Step or Stride Length); (IV) Gait Durations (Step or Stride Length); (V) Stance; (VI) Swing; (VII) Single-Limb Support; (VIII) Double-Limb Support; (IX) Step Width; (X) Gait Asymmetry; (XI) Gait Variability.
Results
A total of 1,989 relevant studies were identified from a database search of PubMed, Medline and Embase. One thousand two hundred ninety-eight studies remained after removal of duplicates. These were screened (by abstract and title) and assessed (by full-text review) for eligibility independently by 2 reviewers (PN, RDF), with a third reviewer consulted until consensus reached (MM). One hundred two hundred fifty-one studies were excluded by screening, 30 studies removed by full-text review, leaving a final 17 included studies for qualitative synthesis in the present narrative review (Appendix 1).
Of the 17 relevant studies included in the review, 5 studies investigated gait patterns in LSS, 10 studies investigated LBP and 2 studies investigated LDH. Of these, 4 studies employed wearable accelerometry in LSS (2 studies) and LBP (2 studies).
Discussion
A tabulation of statistically significant findings from included studies suggests LSS, LBP and LDH have unique patterns of gait deterioration (Table 2). LSS is associated with reduced gait velocity, and gait (step or stride) lengths along with slightly increased gait durations. However, the most marked changes are increases to gait asymmetry and variability. Changes to durations of swing, stance and limb-support phases in LSS have not been studied. Conversely, LDH is characterised by marked reductions to gait velocity, cadence and increases to gait variability, single-limb support and double-limb support. Moderate decreases to gait lengths and moderate increases to swing times, and gait durations are also present. Changes to step width and gait asymmetry have not been studied previously. Gait patterns in LBP involve moderate increases to double-limb support time and step width, with moderate reductions to gait velocity. These changes are accompanied by slightly increased gait durations, swing time, and single-limb support time with slightly decreased gait lengths.
Table 2
| Gait velocity | Cadence | Gait lengths | Gait durations | Swing | Single-limb support | Double-limb support | Step width | Gait asymmetry | Gait variability | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lumbar spinal stenosis | ↓ | ↘ | ↓ | ↗ | X | X | X | X | ↑↑ | ↑↑ |
| Lumbar disc herniation | ↓↓ | ↓↓ | ↓ | ↑ | ↑ | ↑↑ | ↑↑ | X | X | ↑↑ |
| Low back pain | ↓ | ↘ | ↘ | ↗ | ↗ | ↗ | ↑ | ↑ | X | ~ |
Included studies investigating spatiotemporal changes at self-selected and unobstructed walking speeds. ↓↓, markedly decreased (>50%); ↓, decreased (10–50%); ↘, slightly decreased (0–10%); ↗, slightly increased (0–10%); ↑, increased (10–50%); ↑↑, markedly increased (>50%), ~, no significant findings; X, not studied. SDL, Stride Length; SDT, Stride Time.
LSS
Gait deterioration in LSS patients (compared to healthy participants) involves markedly decreased gait velocity and step length together with slightly decreased cadence and slightly increased step duration (Table 3) (10,15-18). Moreover, as ratios of double-limb support (+23%) and stance (+5%) phases increase, swing phase ratio decreases (−8%). Between-feet gait asymmetry is also increased during all phases including stance (+131%), swing (+170%) and double-limb support (+9%) (15). Further, LSS patients also demonstrate greater stride-to-stride gait variability (17). These altered gait patterns likely arise as compensatory adjustments to radicular pain, muscle weakness, (and therefore) low walking tolerance and instability that are exacerbated on upright walking posture (lumbar extension) (19).
Table 3
| Gait velocity | Cadence | Gait length | Gait duration | Gait asymmetry | Gait variability | |
|---|---|---|---|---|---|---|
| Loske et al. (2018) | −16% | – | −12% (SL) | +8% (ST) | +131% (ST) | X |
| Odonkor et al. (2020) | −15% | −10% | −14% (SDL) | X | X | X |
| Papadakis (2009) | X | X | X | X | X | +436%† |
| Perring et al. (2020) | −37% | −14% | −24% (SL) | +16% (ST) | X | X |
| Sun et al. (2018) | −12% | – | −14% (SL) | – | X | X |
| Gait pattern | ↓ | ↘ | ↓ | ↗ | ↑↑ | ↑↑ |
Included studies investigating spatiotemporal changes at self-selected and unobstructed walking speeds. †, signal processing variable; ↓, decreased (10–50%); ↘, slightly decreased (0–10%); ↗, slightly increased (0–10%); ↑↑, markedly increased (>50%); †, signal processing variable; –, no significant findings; X, not studied. ST, step time; SL, step length.
Intermittent claudication is typically considered to be characteristic of LSS, implying the importance step length as an objective gait parameter in informing surgical intervention. However, our findings suggest gait asymmetry and gait variability also be relevant (Table 3).
However, psychological factors like Hawthorne effect (20) and reverse white-coat syndrome (21) may influence testing in observed and unfamiliar laboratory settings via greater conscious control of the walking cycle (22). These factors especially affect stride rhythm and may account for Sun et al. (16)and Loske et al.’s (15) lack of significant findings for cadence and step duration.
Although between-group differences in gait parameters are largely consistent amongst these studies, large discrepancies are present in mean group values possibly owing to variations in cohort demographics such as age, ethnicity, gender distribution, comorbidities and disease severity (23). As such, Odonkor et al. (18) additionally reported effect size differences as a more generalisable representation of gait deterioration in LSS.
These gait patterns in LSS were explored by a more novel methodology: wearable accelerometry in Sun et al. (16) and Loske et al. (15) (Table 2), albeit with a few discrepancies. Sun et al. found LSS patients to have no significant difference in stride duration (P=0.858) and cadence (P=0.629) compared to healthy participants (16). These findings contradict those of Loske et al. (15), who found an (8%) increase in stride duration. This discrepancy likely stems from greater statistical power in Loske et al.’s (15) larger sample size (29 LSS and 27 healthy, versus 20 LSS and 12 healthy participants) compared to Sun et al.’s (16) validation study which primarily sought to demonstrate accuracy and reliability. Moreover, both studies were limited in their analysis of gait metrics to basic spatiotemporal measurements, and future wearable accelerometry studies should endeavour to investigate other gait parameters such as durations and ratios of gait phases.
LBP
LBP most notably involves reduced gait velocity (11,24-31) and increased stride width (11,24,25). Many of the other spatiotemporal parameters of gait undergo much more subtle changes involving slightly increased gait durations whilst cadence and gait lengths undergo slight reductions (Table 4). These gait differences (compared to healthy participants) seem to be greatest in moderate (rather than severe) LBP participants according to findings by Demirel et al. (30).
Table 4
| Gait velocity | Cadence | Gait length | Gait duration | Step width | Swing | Stance | Single-limb support | Double-limb support | Gait variability | |
|---|---|---|---|---|---|---|---|---|---|---|
| Barzilay et al. (2016) | −12% | −3% | −9% (SL) | X | X | X | +2% | +3% | X | X |
| Bonab et al. (2020) | −26% | −19% | −9% (SL), −10% (SDL) | +9% (ST), +8% (SDT) | X | +9% | X | X | +16% | X |
| Demirel et al. (2020)* | −13% | – | −13% (ST), −9% (SDL) | – | X | – | – | – | – | X |
| Demirel et al. (2020)** | −5% | X | −7% (ST), −3% (SDL) | X | X | – | – | – | – | X |
| Hamacher et al. (2016) | −10% | X | X | X | X | X | X | X | X | +75% (SDL), +33% (SDT) |
| Henchoz et al. (2015) | −12% | −25% | −15% (SDL) | X | +25% | X | X | – | – | X |
| Hicks et al. (2017) | −13% | X | −9% (SDL) | +6% (ST) | +50% | X | X | X | +14% | X |
| Gombatto et al. (2015) | – | X | – | – | X | X | X | X | X | X |
| Lamoth et al. (2008) | −14% | X | −14% (SDL) | X | – | X | X | X | X | −48% (SDL) |
| Lee et al. (2007) | −19% | X | X | X | X | X | X | X | X | X |
| Taylor et al. (2003) | – | – | −6% (SDL) | X | X | X | X | X | X | X |
| Gait pattern | ↓ | ↘ | ↘ | ↗ | ↑ | ↗ | ↗ | ↗ | ↑ | – |
Included studies investigating spatiotemporal changes at self-selected and unobstructed walking speeds. *, Moderate LBP; **, severe LBP; ↓, decreased (10–50%); ↘, slightly decreased (0–10%); ↗, slightly increased (0–10%); ↑, increased (10–50%); –, no significant findings; X, not studied. SDL, stride length; SDT, stride time.
These gait adaptations likely arise to minimise (anterior-posterior shear) joint forces on the low back and thus relieve pain (32,33). Supporting this ‘guarding’ hypothesis are the correlation analyses by Bonab et al. (29) whereby reductions in gait velocity and cadence explained nearly 70% and 74% of variance in pain as measure by Visual Analogue Scale (VAS). However, contradicting these findings is Gombatto et al.’s (34) report of no significant differences in spatiotemporal variables of gait. A likely explanation is a much younger population sample (mean age =27.85), suggesting gait deterioration in LBP is not consistent across all age groups.
Only slight alterations to swing, stance, single-limb support and double-limb support durations of gait have been reported by some authors (24,28,29). These differences falling shy of statistical significance for other authors like Henchoz et al. (11) and Demirel et al.(30)suggest these gait changes are less prominent in LBP. Similar inconsistent findings have been reported for gait variability in LBP by Lamoth et al. (25) and Hamacher et al. (31). However, this discrepancy may be due to differences in disease-severity with (VAS) pain scores (VAS; 0= no pain, 10= severe pain) ranging from 2.5–4.8 in Lamoth et al. (25) compared to 4 or higher in Hamacher et al. (31).
Gait patterns in LBP may also be influenced by fear avoidance beliefs or kinesiophobia (30) as significant correlations exist with catastrophising and anticipating pain (35,36). As such, when Lamoth et al. (25) controlled for gait speed many of these differences in spatiotemporal gait parameters (except stride width) are diminished. Nonetheless, the presence of these gait patterns at self-selected (free-living) gait speeds has implications to mobility and quality of life, and also clinical relevance in identifying disease-specific gait patterns.
Wearable accelerometers were used to analyse and profile the gait patterns of LBP participants by Henchoz et al. (11) and Hamachar et al. (31), with similar results to prior laboratory-based finding (Table 4). However, at fixed walking speeds these differences ceased to exist aligning with the prior laboratory-based findings of Hicks et al. (24). Detrembleur et al. (37) hypothesises that musculoskeletal diseases (such as LBP) induce lower-level gait changes compared to neurological pathologies (such as LDH and LSS). Hence, it is plausible that the motor control changes in LBP are too minimal for detection by currently used methodologies–wearable accelerometry-based and laboratory-based techniques alike.
LDH
LDH results in consistent increases in temporal parameters (gait cycle durations, double-limb support and swing duration) and consistent decreases in spatial parameters (gait cycle lengths, gait velocity, and cadence) of gait when compared to healthy controls (Table 5) (29,38). According to findings by Keklicek et al. (38), LDH also involves increased stride-to-stride variability in step lengths. These gait adaptions likely arise as a protective response to sciatic pain seeking to limit hip and spine movement (12,39). Similar pain-avoidance behaviours to LBP patients likely emerge in LDH due to overlapping symptoms (but of greater intensity) and pain-related fears.
Table 5
| Gait velocity | Cadence | Gait length | Gait duration | Swing | Double-limb support | Gait variability | |
|---|---|---|---|---|---|---|---|
| Bonab et al. (2020) | −76% | −67% | −25% (SL), −26% (SDL) | +23% (ST), +28% (SDT) | +23% | +51% | X |
| Keklicek et al. (2018) | X | X | −46% (SL) | X | X | X | +86% (SL) |
| Gait pattern | ↓↓ | ↓↓ | ↓ | ↑ | ↑ | ↑↑ | ↑↑ |
Included studies investigating spatiotemporal changes at self-selected and unobstructed walking speeds. ↓↓, markedly decreased (>50%); ↓, decreased (10–50%); ↑, increased (10–50%); ↑↑, markedly increased (>50%); X, not studied. SDL, stride length; SDT, stride time.
However, contradicting these findings is Huang et al.’s (12) report of increased pelvic rotations enabling normal stride lengths to thereby conserve the energy losses that occur with smaller steps (40). However, this discrepancy may be attributable to Huang et al.’s experimental protocol of controlled walking speed and stride length.
Limitations
Most included studies employed laboratory-based methodologies such as electronic walkways or 3D motion capture systems which are considered the gold standard for precise quantitative gait analysis (41). However, these tracking methods require specialised equipment, trained personnel and travel to an appropriate facility thus being far too expensive, time-consuming and cumbersome for clinical use. Further, these methods may also not accurately reflect real-life and routine walking behaviours (42,43). According to Brodie et al. (43), laboratory assessments overestimate cadence (8.91%, P<0.001) and underestimate gait variability (81.55%, P<0.001) when compared to ‘free-living’ gait in home and community environments. These discrepancies limit clinical use. Additionally, variations in normative gait from individual to individual, may need to be controlled for with age and sex-matched controls.
However, some studies utilised single-point accelerometers (11,15,16,31), formally termed inertial measurement units (IMUs) which have more recently have become a portable and inexpensive means of gait analysis. Through continuous and long-term monitoring these wearable devices more accurately reflect free-living gait (43). They also demonstrate good agreement with traditional gait analysis systems (44). Despite these benefits, IMUs suffer from issues with patient compliance and are prone to drift errors and noise due to interference from amplified mechanical motions and the magnetic fields of other devices (45). These factors can limit data collection rates to 70–90% for typical health monitoring according to findings by Merilahti et al. (46).
Future research
To best of the authors’ knowledge, no studies have investigated gait deterioration in LDH with wearable accelerometry. Huang et al. and Bonab et al. have revealed uniquely different gait patterns in LDH patients but these have been instrumented gait analyses (12,29). Future studies should seek to investigate the ‘free-living’ gait of this population subset using wearable accelerometry in conjunction with other comparable measures.
Further, a comparison of gait patterns to differentiate between degenerative diseases of the lumbar spine has only been conducted by Bonab et al. (29), who examined the gait patterns of LDH, LBP patients and healthy participants. Despite included studies demonstrating significantly altered gait patterns in LDH, LSS and LBP, other gait parameters beyond spatiotemporal metrics such as gait phases, asymmetry and variability have been largely omitted (Table 2). Moreover sensitivity/specificity analyses to discriminate between healthy, LDH and LBP participants was not performed. Moreover, results derived from Bonab et al.’s “WIN-TRACK” instrumented walkway for gait analysis likely reflects the individual’s ‘best’ performance rather than ‘free-living’ gait (29). This may arise due to participants focussing more on walking in standardised laboratory settings when electronic walkways, passive marker systems or motion-capture systems are used, when compared to the results of unobserved monitoring using a discrete wearable device.
Most existing studies of the lumbar spine did not analyse discriminative performance of gait metrics in distinguishing pathological gait patterns from normative gait. This is with the (only) exception of Bidabadi et al. who developed and assessed machine learning algorithms with measured gait parameters in participants with L5 radiculopathy associated dorsiflexion weakness or ‘foot-drop’ achieving a classification accuracy of 93.18% (AUC =0.97) (47). These studies have frequently been conducted in other spinal disease patient populations such as spondylarthritis (48), spinal cord injury (49) and other disease populations including dementia (50), Parkinson’s disease (51,52), stroke (53,54) and musculoskeletal conditions (55,56). Thus, future studies should investigate the diagnostic utility of gait metrics that differ significantly between healthy participants and participants with lumbar spine diseases. Such studies may pave the way for wearable sensor-based gait analysis and objective gait metrics to be implemented in spine care.
Conclusions
Previous studies have highlighted differing patterns of gait deterioration in degenerative diseases of the lumbar spine, albeit with gaps in knowledge (Table 2). While asymmetry and variability are the most distinguishing gait variables in LSS, reduced gait velocity and cadence are present in LDH. Slight spatial and temporal changes are present in LBP. Most of these studies have used laboratory-based methods likely reflecting the individual’s ‘best’ performance, with few studies using discrete wearable devices to capture ‘free-living’ gait. Whilst emerging in other patient populations, diagnostic performance of wearable accelerometry-derived gait metrics is not commonly assessed in participants with degenerative diseases of the lumbar spine.
Acknowledgments
The NeuroSpine Surgery Research Group (NSURG) aided with manuscript production. The Neuro Spine Clinic, Sydney, Australia, provided clinic assistance for authors.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Spine Surgery for the series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-21-91/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-91/coif). The series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” was commissioned by the editorial office without any funding or sponsorship. RJM served as the unpaid Guest Editor of the series and serves as the Editor-in-Chief of Journal of Spine Surgery. RDF and PN served as the unpaid Guest Editors of the series and serve as unpaid Assistant Managing Editors of Journal of Spine Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968-74. [Crossref] [PubMed]
- Luoma K, Riihimäki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487-92. [Crossref] [PubMed]
- Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34:934-40. [Crossref] [PubMed]
- Ravindra VM, Senglaub SS, Rattani A, et al. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Global Spine J 2018;8:784-94. [Crossref] [PubMed]
- Miscusi M, Serrao M, Conte C, et al. Spatial and temporal characteristics of the spine muscles activation during walking in patients with lumbar instability due to degenerative lumbar disk disease: Evaluation in pre-surgical setting. Hum Mov Sci 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Maldaner N, Sosnova M, Zeitlberger AM, et al. Responsiveness of the self-measured 6-minute walking test and the Timed Up and Go test in patients with degenerative lumbar disorders. J Neurosurg Spine 2021; [Epub ahead of print]. [PubMed]
- Maldaner N, Sosnova M, Zeitlberger AM, et al. Evaluation of the 6-minute walking test as a smartphone app-based self-measurement of objective functional impairment in patients with lumbar degenerative disc disease. J Neurosurg Spine 2020;33:779-88. [Crossref] [PubMed]
- Perry J, Davids JR. Gait analysis: normal and pathological function. J Pediatr Orthop 1992;12:815. [Crossref]
- Gor-García-Fogeda MD, Cano de la Cuerda R, Carratalá Tejada M, et al. Observational Gait Assessments in People With Neurological Disorders: A Systematic Review. Arch Phys Med Rehabil 2016;97:131-40. [Crossref] [PubMed]
- Perring J, Mobbs R, Betteridge C. Analysis of Patterns of Gait Deterioration in Patients with Lumbar Spinal Stenosis. World Neurosurg 2020;141:e55-9. [Crossref] [PubMed]
- Henchoz Y, Soldini N, Peyrot N, et al. Energetics and mechanics of walking in patients with chronic low back pain and healthy matched controls. Eur J Appl Physiol 2015;115:2433-43. [Crossref] [PubMed]
- Huang YP, Bruijn SM, Lin JH, et al. Gait adaptations in low back pain patients with lumbar disc herniation: trunk coordination and arm swing. Eur Spine J 2011;20:491-9. [Crossref] [PubMed]
- Mirelman A, Shema S, Maidan I, et al. Gait. Handb Clin Neurol 2018;159:119-34. [Crossref] [PubMed]
- Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson's disease? Towards an optimal testing protocol. Gait Posture 2013;37:580-5. [Crossref] [PubMed]
- Loske S, Nüesch C, Byrnes KS, et al. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. Spine J 2018;18:2195-204. [Crossref] [PubMed]
- Sun J, Liu YC, Yan SH, et al. Clinical Gait Evaluation of Patients with Lumbar Spine Stenosis. Orthop Surg 2018;10:32-9. [Crossref] [PubMed]
- Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas 2009;30:1171-86. [Crossref] [PubMed]
- Odonkor C, Kuwabara A, Tomkins-Lane C, et al. Gait features for discriminating between mobility-limiting musculoskeletal disorders: Lumbar spinal stenosis and knee osteoarthritis. Gait Posture 2020;80:96-100. [Crossref] [PubMed]
- Suda Y, Saitou M, Shibasaki K, et al. Gait analysis of patients with neurogenic intermittent claudication. Spine (Phila Pa 1976) 2002;27:2509-13. [Crossref] [PubMed]
- Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ 2015;351:h4672. [Crossref] [PubMed]
- Larkin KT, Schauss SL, Elnicki DM, et al. Detecting white coat and reverse white coat effects in clinic settings using measures of blood pressure habituation in the clinic and patient self-monitoring of blood pressure. J Hum Hypertens 2007;21:516-24. [Crossref] [PubMed]
- Hillel I, Gazit E, Nieuwboer A, et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur Rev Aging Phys Act 2019;16:6. [Crossref] [PubMed]
- Ebersbach G, Sojer M, Müller J, et al. Sociocultural differences in gait. Mov Disord 2000;15:1145-7. [Crossref] [PubMed]
- Hicks GE, Sions JM, Coyle PC, et al. Altered spatiotemporal characteristics of gait in older adults with chronic low back pain. Gait Posture 2017;55:172-6. [Crossref] [PubMed]
- Lamoth CJ, Stins JF, Pont M, et al. Effects of attention on the control of locomotion in individuals with chronic low back pain. J Neuroeng Rehabil 2008;5:13. [Crossref] [PubMed]
- Lee CE, Simmonds MJ, Etnyre BR, et al. Influence of pain distribution on gait characteristics in patients with low back pain: part 1: vertical ground reaction force. Spine (Phila Pa 1976) 2007;32:1329-36. [Crossref] [PubMed]
- Taylor NF, Evans OM, Goldie PA. The effect of walking faster on people with acute low back pain. Eur Spine J 2003;12:166-72. [Crossref] [PubMed]
- Barzilay Y, Segal G, Lotan R, et al. Patients with chronic non-specific low back pain who reported reduction in pain and improvement in function also demonstrated an improvement in gait pattern. Eur Spine J 2016;25:2761-6. [Crossref] [PubMed]
- Bonab M, Colak TK, Toktas ZO, et al. Assessment of Spatiotemporal Gait Parameters in Patients with Lumbar Disc Herniation and Patients with Chronic Mechanical Low Back Pain. Turk Neurosurg 2020;30:277-84. [PubMed]
- Demirel A, Onan D, Oz M, et al. Moderate disability has negative effect on spatiotemporal parameters in patients with chronic low back pain. Gait Posture 2020;79:251-5. [Crossref] [PubMed]
- Hamacher D, Hamacher D, Krowicki M, et al. Gait Variability in Chronic Back Pain Sufferers With Experimentally Diminished Visual Feedback: A Pilot Study. J Mot Behav 2016;48:205-8. [Crossref] [PubMed]
- Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech (Bristol, Avon) 1999;14:203-16. [Crossref] [PubMed]
- Seay JF, Van Emmerik RE, Hamill J. Influence of low back pain status on pelvis-trunk coordination during walking and running. Spine (Phila Pa 1976) 2011;36:E1070-9. [Crossref] [PubMed]
- Gombatto SP, Brock T, DeLork A, et al. Lumbar spine kinematics during walking in people with and people without low back pain. Gait Posture 2015;42:539-44. [Crossref] [PubMed]
- Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, et al. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res 2003;26:101-8. [PubMed]
- Pakzad M, Fung J, Preuss R. Pain catastrophizing and trunk muscle activation during walking in patients with chronic low back pain. Gait Posture 2016;49:73-7. [Crossref] [PubMed]
- Detrembleur C, van den Hecke A, Dierick F. Motion of the body centre of gravity as a summary indicator of the mechanics of human pathological gait. Gait Posture 2000;12:243-50. [Crossref] [PubMed]
- Keklicek H, Demirel A, Kirdi E, et al. P 017 - Gait stability in patient with lumbar herniation nucleus pulposus. Gait Posture 2018;65:258-9. [Crossref]
- Mannion AF, Dvorak J, Müntener M, et al. A prospective study of the interrelationship between subjective and objective measures of disability before and 2 months after lumbar decompression surgery for disc herniation. Eur Spine J 2005;14:454-65. [Crossref] [PubMed]
- Bertram JE, Ruina A. Multiple walking speed-frequency relations are predicted by constrained optimization. J Theor Biol 2001;209:445-53. [Crossref] [PubMed]
- Schniepp R, Möhwald K, Wuehr M. Clinical and automated gait analysis in patients with vestibular, cerebellar, and functional gait disorders: perspectives and limitations. J Neurol 2019;266:118-22. [Crossref] [PubMed]
- Simon SR. Quantification of human motion: gait analysis-benefits and limitations to its application to clinical problems. J Biomech 2004;37:1869-80. [Crossref] [PubMed]
- Brodie MA, Coppens MJ, Lord SR, et al. Wearable pendant device monitoring using new wavelet-based methods shows daily life and laboratory gaits are different. Med Biol Eng Comput 2016;54:663-74. [Crossref] [PubMed]
- Petraglia F, Scarcella L, Pedrazzi G, et al. Inertial sensors versus standard systems in gait analysis: a systematic review and meta-analysis. Eur J Phys Rehabil Med 2019;55:265-80. [Crossref] [PubMed]
- Felisberto F, Fdez-Riverola F, Pereira A. A ubiquitous and low-cost solution for movement monitoring and accident detection based on sensor fusion. Sensors (Basel) 2014;14:8961-83. [Crossref] [PubMed]
- Merilahti J, Pärkkä J, Antila K, et al. Compliance and technical feasibility of long-term health monitoring with wearable and ambient technologies. J Telemed Telecare 2009;15:302-9. [Crossref] [PubMed]
- Sharif Bidabadi S, Murray I, Lee GYF, et al. Classification of foot drop gait characteristic due to lumbar radiculopathy using machine learning algorithms. Gait Posture 2019;71:234-40. [Crossref] [PubMed]
- Soulard J, Vaillant J, Balaguier R, et al. Foot-Worn Inertial Sensors Are Reliable to Assess Spatiotemporal Gait Parameters in Axial Spondyloarthritis under Single and Dual Task Walking in Axial Spondyloarthritis. Sensors (Basel) 2020;20:6453. [Crossref] [PubMed]
- Werner C, Schneider S, Gassert R, et al. Complementing Clinical Gait Assessments of Spinal Cord Injured Individuals using Wearable Movement Sensors. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:3142-5. [Crossref] [PubMed]
- Mc Ardle R, Del Din S, Galna B, et al. Differentiating dementia disease subtypes with gait analysis: feasibility of wearable sensors? Gait Posture 2020;76:372-6. [Crossref] [PubMed]
- Khoury N, Attal F, Amirat Y, et al. Data-Driven Based Approach to Aid Parkinson's Disease Diagnosis. Sensors (Basel) 2019;19:242. [Crossref] [PubMed]
- Moon S, Song HJ, Sharma VD, et al. Classification of Parkinson's disease and essential tremor based on balance and gait characteristics from wearable motion sensors via machine learning techniques: a data-driven approach. J Neuroeng Rehabil 2020;17:125. [Crossref] [PubMed]
- Hsu WC, Sugiarto T, Lin YJ, et al. Multiple-Wearable-Sensor-Based Gait Classification and Analysis in Patients with Neurological Disorders. Sensors (Basel) 2018;18:3397. [Crossref] [PubMed]
- Zhang W, Smuck M, Legault C, et al. Gait Symmetry Assessment with a Low Back 3D Accelerometer in Post-Stroke Patients. Sensors (Basel) 2018;18:3322. [Crossref] [PubMed]
- Tedesco S, Crowe C, Ryan A, et al. Motion Sensors-Based Machine Learning Approach for the Identification of Anterior Cruciate Ligament Gait Patterns in On-the-Field Activities in Rugby Players. Sensors (Basel) 2020;20:3029. [Crossref] [PubMed]
- Tesconi M, Pasquale Scilingo E, Barba P, et al. Wearable kinesthetic system for joint knee flexion-extension monitoring in gait analysis. Conf Proc IEEE Eng Med Biol Soc 2006;2006:1497-500. [Crossref] [PubMed]


