
Inter-bout and intra-bout gait variability—proposed objective measures of gait deterioration during prolonged walking in spine care
Walking analysis using wearable devices
Walking is a fundamental part of independent living and relies on a complex interplay between a person’s neurological and musculoskeletal systems. Alterations to walking patterns can occur in spinal pathologies such as lumbar disc herniation (1-3), lumbar spinal stenosis (LSS) (4-6), mechanical low back pain (MLBP) (1), and cervical myelopathy (7). In these spinal diseases, walking has been shown to be a relevant biomarker of both decline and recovery (8).
Wearable devices (such as smartphones, smartwatches, and activity trackers) can contain microelectromechanical sensors such as accelerometers, gyroscopes, and magnetometers. These sensors can objectively quantify walking patterns using metrics such as step count, walking speed, cadence, and step length (9). Wearable devices can be worn at a single point on the body (such as the wrist, sternum, lower back, or ankle) or at multiple points (though single-point wearable sensors may have greater clinical utility due to being less obtrusive) (10). Being small, cheap, and convenient, wearable devices can be taken home by people into their everyday environment, allowing for continuous remote monitoring without the Hawthorne effect (where an individual’s walking patterns may altered due to the awareness of being observed by a clinician or laboratory personnel) (11-14).
Objective measurement of gait deterioration
There is little agreement in the literature regarding an objective metric for the quantification of gait deterioration (or improvement) over time, and, to our knowledge, almost no objective exploration of this concept in the field of spine health.
Within a walking bout
Gait deterioration may occur over a single walking bout, as is the case in patients with LSS. LSS is classically associated with neurogenic claudication, a clinical syndrome of back or leg pain, weakness, and paraesthesia that worsens with prolonged walking—causing patients to walk slower and “hunch” their backs over the course of a walking bout (15). Nagai et al. attempted to quantify gait deterioration in LSS patients by comparing their gait using wearable sensors at the beginning of their walking bout and at the end (when patients expressed walking difficulty) (16). Patients had slower walking speeds by the end of the bout (1.01 vs. 0.96 m/s, though this did not reach statistical significance with the study’s small sample size of 12 patients), and significantly worse postural sway (P<0.05). However, when measuring walking deterioration between the start and end of each patient’s walking bout, comparisons between patients are complicated as each patient walked for a unique distance. Interestingly, there have been attempts to objectively quantify walking deterioration in patients with multiple sclerosis (17-19). Engelhard et al. introduced the Warp Score and found increases (signifying deterioration) in the Warp Score by a mean of about three points over six minutes of walking (17). While acting as a continuous and objective measure of walking deterioration, this score is not easily replicable (involving dynamic time warping, an algorithm for curve registration) for the purpose of routine clinical use.
Across different walking bouts
On the other hand, gait deterioration can occur across walking bouts over the course of a day. Patients with spinal disorders frequently report variation in symptom intensity throughout the day. Patients with MLBP due to osteoarthritic changes (1,20) typically report worsening symptoms over the day with increased joint use, whilst patients with low back pain due to inflammatory processes (21,22) report morning stiff that alleviates with activity during the day. To our knowledge, no studies in the field of spine care have investigated how gait deterioration with low back pain (23) varies over the course of a day. Again, some studies have investigated this concept using patients with multiple sclerosis (24,25). First investigated by Morris et al., no clinically significant differences were found between five-hour interval gait trials in terms of gait speed, cadence, stride length and double limb support duration (24). Later investigated by Jacob et al., significant time of day dependent gait changes were once again not detected in terms of both 6MWT performance (299.98 vs. 293.39 m, P=0.237) and gait speed (0.71 vs. 0.69 m/s, P=0.385) (25). However, these studies measured gait deterioration at distinct snapshots. Continuous tracking of ‘free-living’ gait metrics may be more suitable in holistically capturing holistic fluctuations in gait over the course of a day.
Intra- and inter-bout gait variability
To objectively quantify these facets of gait deterioration, we propose a framework leveraging the capabilities of wearable devices to perform continuous capture of gait metrics:
- Intra-bout gait variability: continuous tracking of gait deterioration within a single walking bout;
- Inter-bout gait variability: continuous tracking of gait deterioration when comparing walking bouts over the course of a day.
One way of objectively and continuously measuring intra-bout gait deterioration is by graphical representation (as a gait variability curve) of fluctuations in specific components of gait such as gait velocity and cadence, as we have done in Figures 1,2. However, besides obvious differences, this graphical approach is likely difficult to compare between patients, and future studies may look to instead propose simple summary scores (for example, out of 100) to capture the extent of inter- or intra-bout gait deterioration (or regularity in the normative population).
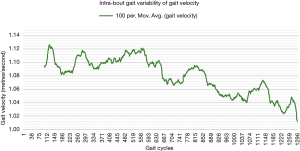
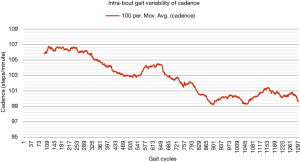
Future directions
Healthy patients would also be expected to experience at least some gait deterioration during prolonged walking. Pathological gait deterioration during prolonged walking can only be appreciated after normal gait deterioration is investigated. To our knowledge, no study has investigated normal gait deterioration in prolonged walking, marking a large space of research potential in this area.
Once paired with wearable sensors which can collect continuous gait and walking data from patients in their everyday environment, we envision that both intra- and inter-bout gait variability will be useful in the identification and monitoring of disease. These diseases may include but are not limited to hip and knee osteoarthritis, spinal pathologies, Parkinson’s disease, motor neuron disease, myopathies, and cardiorespiratory disorders. Future research can be focused towards analysing intra- and inter-bout variability trends across different diseases, such that a patient’s unique intra- and inter-bout variability can be matched with disease-specific patterns to aid in clinical decision-making, the assessment of disability, and potentially pathology recognition with artificial intelligence assistance. In addition, once disease is established, changes in a patient’s intra- and inter-bout variability could indicate an improvement or deterioration in symptoms and quantify the benefit of any intervention.
Acknowledgments
The NeuroSpine Clinic aided with recruitment of our example patient, Mr. R. The Wearables and Gait Analysis Research Group provided the wearable sensor used to evaluate our example patient.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Spine Surgery for the series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient”. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-88/coif). The series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” was commissioned by the editorial office without any funding or sponsorship. RJM served as the unpaid Guest Editor of the series and serves as the Editor-in-Chief of Journal of Spine Surgery. RDF and PN served as the unpaid Guest Editors of the series and serve as unpaid Assistant Managing Editors of Journal of Spine Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonab M, Colak TK, Toktas ZO, et al. Assessment of Spatiotemporal Gait Parameters in Patients with Lumbar Disc Herniation and Patients with Chronic Mechanical Low Back Pain. Turk Neurosurg 2020;30:277-84. [PubMed]
- Zheng CF, Liu YC, Hu YC, et al. Correlations of Japanese Orthopaedic Association Scoring Systems with Gait Parameters in Patients with Degenerative Spinal Diseases. Orthop Surg 2016;8:447-53. [Crossref] [PubMed]
- Ghent F, Mobbs RJ, Mobbs RR, et al. Assessment and Post-Intervention Recovery After Surgery for Lumbar Disk Herniation Based on Objective Gait Metrics from Wearable Devices Using the Gait Posture Index. World Neurosurg 2020;142:e111-6. [Crossref] [PubMed]
- Grelat M, Gouteron A, Casillas JM, et al. Walking Speed as an Alternative Measure of Functional Status in Patients with Lumbar Spinal Stenosis. World Neurosurg 2019;122:e591-7. [Crossref] [PubMed]
- Mobbs RJ, Mobbs RR, Choy WJ. Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: the Gait Posture index (GPi). J Spine Surg 2019;5:300-9. [Crossref] [PubMed]
- Chakravorty A, Mobbs RJ, Anderson DB, et al. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: systematic review. BMC Musculoskelet Disord 2019;20:288. [Crossref] [PubMed]
- Haddas R, Lieberman I, Arakal R, et al. Effect of Cervical Decompression Surgery on Gait in Adult Cervical Spondylotic Myelopathy Patients. Clin Spine Surg 2018;31:435-40. [Crossref] [PubMed]
- Mobbs RJ. Gait velocity (walking speed) is an indicator of spine health, and objective measure of pre and post intervention recovery for spine care providers. J Spine Surg 2020;6:353-5. [Crossref] [PubMed]
- Tao W, Liu T, Zheng R, et al. Gait analysis using wearable sensors. Sensors (Basel) 2012;12:2255-83. [Crossref] [PubMed]
- Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219-29. [Crossref] [PubMed]
- Robles-García V, Corral-Bergantiños Y, Espinosa N, et al. Spatiotemporal Gait Patterns During Overt and Covert Evaluation in Patients With Parkinson’s Disease and Healthy Subjects: Is There a Hawthorne Effect? J Appl Biomech 2015;31:189-94. [Crossref] [PubMed]
- Ardestani MM, Hornby TG. Effect of investigator observation on gait parameters in individuals with stroke. J Biomech 2020;100:109602. [Crossref] [PubMed]
- Vickers J, Reed A, Decker R, et al. Effect of investigator observation on gait parameters in individuals with and without chronic low back pain. Gait Posture 2017;53:35-40. [Crossref] [PubMed]
- Amin T, Mobbs RJ, Mostafa N, et al. Wearable devices for patient monitoring in the early postoperative period: a literature review. Mhealth 2021;7:50. [Crossref] [PubMed]
- Lee SY, Kim TH, Oh JK, et al. Lumbar Stenosis: A Recent Update by Review of Literature. Asian Spine J 2015;9:818-28. [Crossref] [PubMed]
- Nagai K, Aoyama T, Yamada M, et al. Quantification of changes in gait characteristics associated with intermittent claudication in patients with lumbar spinal stenosis. J Spinal Disord Tech 2014;27:E136-42. [Crossref] [PubMed]
- Engelhard MM, Dandu SR, Patek SD, et al. Quantifying six-minute walk induced gait deterioration with inertial sensors in multiple sclerosis subjects. Gait Posture 2016;49:340-5. [Crossref] [PubMed]
- Taborri J, Studer V, Grossi P, et al. Reliability and Repeatability Analysis of Indices to Measure Gait Deterioration in MS Patients during Prolonged Walking. Sensors (Basel) 2020;20:5063. [Crossref] [PubMed]
- Shema-Shiratzky S, Gazit E, Sun R, et al. Deterioration of specific aspects of gait during the instrumented 6-min walk test among people with multiple sclerosis. J Neurol 2019;266:3022-30. [Crossref] [PubMed]
- Najafi S, Rezasoltani Z, Abedi M. Effects of mechanical low back pain in spatiotemporal parameters of gait. J Arch Mil Med 2018;6:e82816.
- Soulard J, Vaillant J, Agier CT, et al. Gait characteristics in patients with ankylosing spondylitis: a systematic review. Clin Exp Rheumatol 2021;39:173-86. [PubMed]
- Del Din S, Carraro E, Sawacha Z, et al. Impaired gait in ankylosing spondylitis. Med Biol Eng Comput 2011;49:801-9. [Crossref] [PubMed]
- Barzilay Y, Segal G, Lotan R, et al. Patients with chronic non-specific low back pain who reported reduction in pain and improvement in function also demonstrated an improvement in gait pattern. Eur Spine J 2016;25:2761-6. [Crossref] [PubMed]
- Morris ME, Cantwell C, Vowels L, et al. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2002;72:361-5. [Crossref] [PubMed]
- Jacob A. The effect of time of day, the test-retest reliability and the relationship of gait outcome measures with fatigue in persons with multiple sclerosis. 2018. Available online: https://eresearch.qmu.ac.uk/handle/20.500.12289/9511


