
Towards personalized and value-based spine care: objective patient monitoring with smartphone activity data
Quantification of patient outcomes has been a cornerstone of evidence-based medicine since its inception. This concept of evaluating the efficacy of a medical intervention by assessing patient health and clinical outcomes continually drives physicians to develop measures that accurately portray a patient’s true physiologic state. However, clinical outcome measures in spine surgery are often sparse and subjective. We suggest that mobility data obtained from patients’ smartphones is a useful metric for assessing patients’ clinical improvement and can be used as an objective outcome measure in spine surgery. Objective patient monitoring also paves the way for value-based spine care, whereby surgery efficacy is determined by the degree of improvement in the patient’s health and quality of life.
Current outcome measures in spine surgery
The most commonly employed type of clinical outcome metric in spine surgery is patient reported outcome measures (PROMs). The inception of PROMs in spine surgery dates back to 1978, when Lee et al. commented “A standard evaluation system for functional disabilities in patients with chronic low-back pain is necessary for determination of the level of incapacitation (disability) and for comparison of treatment results” (1). Because objective assessment of patient mobility was not possible at the time, the authors created a subjective, patient-reported evaluation system that assessed patient mobility by asking the patient to respond to questions regarding ease of ambulation, maximal walking distance, and estimated sitting time. The authors’ questionnaire effectively stratified satisfactory and unsatisfactory surgical results in patients who underwent decompression for spinal stenosis, showcasing the first correlation of mobility measures with surgical outcome.
This initial study catalyzed a gradual shift in spine surgery outcomes measurement, away from solely objective physical factors such as motor exam findings and sensory changes, and instead emphasizing patients’ perspective of their health and functioning (2). The development of numerous PROMs confirms this trend (3). Instruments such as the Oswestry disability index (ODI) (4), visual analog scale (VAS) (5), EuroQOL-5D (EQ-5D) (6), and 36-item short form health survey (SF-36) (7), all rely on self-reported static questionnaires, a methodology that has largely remained unchanged since its inception over 40 years ago. These and other PROMs are widely used in the literature to assess patient disability, pain, and/or quality of life after surgical intervention, and generally satisfy the three major evaluation criteria for outcome assessment: validity (i.e., how accurately an instrument measures what it intends to measure), reliability (i.e., reproducibility), and responsiveness (i.e., ability to detect change) (8).
Drawbacks of PROMs
Despite the usefulness of PROMs with regards to outcomes measurement, they remain inadequate due to the following limitations.
The greatest drawback of PROMs in their current form is their inherently subjective and discrete nature. Questionnaires relying on patients’ recollection of their general state of health can be weakened by recall bias. While no studies have specifically examined this effect in the context of spine surgery, several investigations have found that recall bias from similar PROMs can significantly impact clinical decision making, as variability between repeated survey administrations approaches the established minimum clinically important difference (9-11). Additionally, PROMs currently require the patient to be in-office when filling out the survey and thus only provide data at discrete time points. This further amplifies the potential inaccuracy from self-reporting for chronic conditions such as pain, as patient responses can be skewed based on mood or symptom severity on the day of their clinic visit (12,13). In fact, some PROMs, such as the EQ-5D, specifically patients to answer questions based on their health on the day of the survey, which precludes a robust characterization of patient outcomes across time. Even when PROM surveys are designed to estimate longitudinal quality of life, oftentimes —except in the context of clinical trials—surveys may only be conducted once before and once after intervention, or only once in the post-operative setting (without ascertaining patients’ baseline for comparison). Again, this hinders surgeons from obtaining a true understanding of patients’ functional health and wellbeing.
Furthermore, the comparability and reliability of PROMs is suboptimal. Reliability of surveys correlates with survey length, with longer surveys having lower completion rates and thus lower quality data (14,15). This results in the derivation of vastly different utility values for the same condition depending on the employed instrument, limiting comparability (16). Studies also indicate that response fatigue can arise during the course of a single survey, with less detail and attention given to questions answered at the end of a survey compared to the beginning (17).
Lastly, and perhaps most significantly, is the high financial burden of incorporating PROMs into a clinical practice. Licensing and data entry can cost upwards of $150,000 per year. PROM implementation is also time-consuming for patients and office staff, and was cited as a major reason for why an estimated 32% of spine surgeons in all practice settings (academic, public, private) across North America, Latin America, Europe, Asia, and the Middle East elect not to use PROM questionnaires routinely (18).
Objective activity tracking as a clinical outcome measure
While current PROMs are useful in assessing outcome after spine surgery, their limitations warrant development of improved outcome measures with higher accuracy. There is a growing body of literature dedicated to the development and validation of objective outcome measures based on patient mobility. One of the first studies to do so utilized wearable accelerometers that tracked patients’ activity levels, such as measuring steps taken per day (19). This data allows clinicians to assess how active and mobile a patient is, fulfilling the original goal of PROMs—“to obtain standard objective evaluation of functional limitations of patients” (1)—through a more objective and continuous method. Accelerometer-based metrics such as gait velocity and step count are known indicators of well-being in spine patient populations (20,21), and thus this objective activity measurement was an important proof-of-concept that opened the door to mobility tracking as a potential means of assessing post-operative outcome in spine surgery.
The adoption of health-monitoring devices such as smartphones and smartwatches with built-in accelerometer functionality offers an even more complete source of data to gauge patient benefit from surgical interventions. Smartphones have remarkable fidelity in capturing activity data (22), and their retroactive storage can offer a unique window into the course of a patient’s pre-operative decline as well as post-operative recovery. Datapoints are collected up to every hour, giving surgeons the ability to assess patient mobility with fine temporal resolution throughout the entire post-operative period. This is especially advantageous compared to PROMs, which often only gather 2–3 datapoints over the course of a patient’s surgery and recovery. Additionally, passive data collection via smartphone technology obviates the need for active patient participation, which alleviates compliance-related barriers to data collection as noted in studies relying solely on PROMs or accelerometers.
Early investigations utilizing smartphone data for activity monitoring have shown that patient mobility correlates with the expected peri-operative timeline: a decrease in pre-operative activity secondary to, for example, spine pathology; a decrease in activity during the immediate post-operative period as patients recover from surgery; and a gradual increase in activity when compared to baseline (in the case of a successful surgery) (23). This method of data collection and analysis is relatively new, and hence additional studies are needed to quantify its feasibility and validity. Nonetheless, this initial work shows that smartphone-based activity tracking is a promising addition to current subjective and discrete outcome measures such as PROMs.
Our group has built on these preliminary studies and developed a novel algorithm that uses smartphone-based mobility data to automatically classify patients’ pre-operative clinical course as well as post-operative outcome. After conducting a time series analysis on the number of steps-per-day across a 2-year peri-operative window, up to five temporal epochs were identified from each patient’s time series in a data-driven manner: (I) pre-operative baseline; (II) acute pre-operative decline, indicating either an acute event or an acute-on-chronic decline; (III) immediate post-operative recovery; (IV) fully-recovered state; (V) secondary decline from a fully-recovered state (Figure 1).
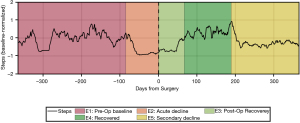
Our findings highlight the additional information that objective outcomes can provide, such as patients’ baseline mobility, the course of post-operative recovery, and the extent of functional improvement after surgery. Defining and quantifying functional state in these peri-operative stages has numerous other implications in pre-operative risk stratification and prediction of expected post-operative mobility outcomes. Further development of our analytic methodology will rely on assessing a larger cohort of patients and comparing these objective outcome measures to validated PROMs scores.
Future directions in objective activity monitoring: personalized and value-based spine care
While still in its nascency, objective activity monitoring can go beyond supplementing PROMs in spine surgery outcome measurement. We posit that smartphone-based activity data has the potential to usher in a new era of personalized, value-based spine care with paradigm shifts in the prognostication and evaluation of spine surgery, as well as in its reimbursement.
A major component of personalized medicine is the crafting of a tailored treatment strategy for each patient based on their individual characteristics. In surgery, this means the data-driven selection of a specific surgical approach, as well as prediction of outcomes for the purposes of patient counseling, incorporating other predictive factors such as demographic and clinical variables. While demographics-based predictive frameworks only explain 32–59% of the variance in spine surgery outcome, the combination with 3-month post-operative PROMs data improved discriminative power by 10% (24,25). Though the addition of PROMs data is beneficial, we believe that the inclusion of continuous mobility measures will further improve predictive power. Effective prognosis is crucial when constructing a treatment plan, as identifying patients who are likely to not improve after spine surgery will save healthcare resources and mitigate patient dissatisfaction in the event of a suboptimal surgical outcome.
For patients in whom surgery is indicated, real-time streaming of patient mobility data can help surgeons actively follow patient recovery. At our institution, post-operative follow-up appointments are scheduled 4–6 weeks after spine surgery, though there is limited literature to specifically endorse this standard. Additionally, while it is well known that the course of recovery varies greatly with demographic characteristics such as patient age (26), BMI (27), and co-morbidities (28), there is no protocol for adjusting the initial follow-up period to account for these baseline differences. This one-size-fits-all approach is inefficient, and thus costly, as patients who are recovering at different speeds are treated homogenously. Instead, in the future it may be possible for surgeons to remotely monitor smartphone-based activity data and customize each patient’s follow-up schedule (e.g., earlier if patient’s recovery is below what is expected).
Remote monitoring of patient activity can also enable earlier identification of unexpected decreases in mobility, which may prompt further medical work-up of the underlying cause (e.g., re-herniations after diskectomy). When such events occur after the initial post-operative follow-up period, a mechanism to identify such patients opens the possibility of proactively initiating neurosurgical care prior to further progression or degeneration.
Along with personalized pre- and post-operative care delivery, objective assessment of surgical outcomes offers data that can be used by providers to further shape the transformation of spine surgery economics towards a value-based care model. Value-based care is a healthcare delivery model where providers, such as physicians and hospitals, are paid based on patient outcomes (29). This departs from a fee-for-service or capitated model, where providers are reimbursed based on the quantity of services delivered.
Improvement in a patient’s post-operative activity is highly indicative of the value that surgery has imparted on that patient. Reimbursing based on the total value derived by patients, rather than by the extensiveness of surgical intervention, re-aligns the healthcare system’s priorities towards providing patients with maximal benefit. Importantly, we would like to emphasize that mobility-based metrics should not be the only factor used to determine reimbursement in this scheme, but would rather be one component of a multi-faceted approach that includes other positive factors that contribute to patient benefit (e.g., post-operative pain reduction, narcotics reduction), as well as negative factors (e.g., complications, re-admission rates).
Conclusions
Smartphone-based activity monitoring offers an objective and continuous source of data that can be used as a proxy for assessing functional improvement in patients following spine surgery. This form of outcome measurement addresses several shortcomings in PROMs—currently the gold standard—such as potential bias from the subjective assessment of patients, temporally discrete collection of data, logistic/financial constraints on data collection, and lack of validity and comparability. In the future, the adoption of objective outcome measures may allow surgeons and hospital systems to practice personalized and value-based care. The quantifiable nature of smartphone-based activity data can allow for better prognostication and surgical decision making, as well as customized patient follow-up with remote monitoring. The accessibility and ease-of-collection of activity data utilizing smartphones has the potential to speed up the implementation of value-based reimbursement across health systems. Additional studies that assess smartphone-based activity monitoring in spine surgery are needed before any of these future possibilities can become a widespread reality. We propose that future studies should focus on validation of mobility-based outcome measures in large cohorts of patients with respect to widely-accepted outcome measures, such as PROMs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by Guest Editors (Ralph J. Mobbs, Pragadesh Natarajan & R. Dineth Fonseka) for the series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” published in Journal of Spine Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-21-67/coif). The series “Objective Monitoring and Wearable Technologies including Sensor-Based Accelerometers and Mobile Health Applications for the Spine Patient” was commissioned by the editorial office without any funding or sponsorship. GWB reports standard stock ownership in Kinesiometrics LLC, outside the submitted work. MYW is a consultant for DePuy Synthes, K2M, Stryker, and Spineology; he is a patent holder with DePuy Synthes; he has direct stock ownership in ISD and Medical Device Partners; and has direct stock ownership in Kinesiometrics LLC; outside the submitted work. JWY reports standard stock ownership in Kinesiometrics LLC and MedCyclops LLC, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee CK, Hansen HT, Weiss AB. Developmental lumbar spinal stenosis. Pathology and surgical treatment. Spine (Phila Pa 1976) 1978;3:246-55. [Crossref] [PubMed]
- Finkelstein JA, Schwartz CE. Patient-reported outcomes in spine surgery: past, current, and future directions. J Neurosurg Spine 2019;31:155-64. [Crossref] [PubMed]
- Nayak NR, Coats JM, Abdullah KG, et al. Tracking patient-reported outcomes in spinal disorders. Surg Neurol Int 2015;6:S490-9. [Crossref] [PubMed]
- Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3. [PubMed]
- Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90-4. [Crossref] [PubMed]
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [Crossref] [PubMed]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [Crossref] [PubMed]
- Ghogawala Z, Resnick DK, Watters WC 3rd, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 2: assessment of functional outcome following lumbar fusion. J Neurosurg Spine 2014;21:7-13. [Crossref] [PubMed]
- Topp J, Andrees V, Heesen C, et al. Recall of health-related quality of life: how does memory affect the SF-6D in patients with psoriasis or multiple sclerosis? A prospective observational study in Germany. BMJ Open 2019;9:e032859. [Crossref] [PubMed]
- Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD 2005;2:91-7. [Crossref] [PubMed]
- Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes 2003;1:4. [Crossref] [PubMed]
- Clarke PM, Fiebig DG, Gerdtham UG. Optimal recall length in survey design. J Health Econ 2008;27:1275-84. [Crossref] [PubMed]
- Stull DE, Leidy NK, Parasuraman B, et al. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin 2009;25:929-42. [Crossref] [PubMed]
- Holland R, Smith RD, Harvey I, et al. Assessing quality of life in the elderly: a direct comparison of the EQ-5D and AQoL. Health Econ 2004;13:793-805. [Crossref] [PubMed]
- Barton GR, Sach TH, Avery AJ, et al. A comparison of the performance of the EQ-5D and SF-6D for individuals aged >or= 45 years. Health Econ 2008;17:815-32. [Crossref] [PubMed]
- Whitehurst DG, Bryan S, Lewis M. Systematic review and empirical comparison of contemporaneous EQ-5D and SF-6D group mean scores. Med Decis Making 2011;31:E34-44. [Crossref] [PubMed]
- Sahlqvist S, Song Y, Bull F, et al. Effect of questionnaire length, personalisation and reminder type on response rate to a complex postal survey: randomised controlled trial. BMC Med Res Methodol 2011;11:62. [Crossref] [PubMed]
- Falavigna A, Dozza DC, Teles AR, et al. Current Status of Worldwide Use of Patient-Reported Outcome Measures (PROMs) in Spine Care. World Neurosurg 2017;108:328-35. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Maharaj M, et al. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Global Spine J 2016;6:459-64. [Crossref] [PubMed]
- Mobbs RJ. Gait velocity (walking speed) is an indicator of spine health, and objective measure of pre and post intervention recovery for spine care providers. J Spine Surg 2020;6:353-5. [Crossref] [PubMed]
- Mobbs RJ, Betteridge C. Daily step count and walking speed as general measures of patient wellbeing. J Spine Surg 2020;6:635-6. [Crossref] [PubMed]
- Apple. Measuring Walking Quality Through iPhone Mobility Metrics. 2020. Available online: https://www.apple.com/in/healthcare/docs/site/Measuring_Walking_Quality_Through_iPhone_Mobility_Metrics.pdf
- Basil GW, Sprau AC, Eliahu K, et al. Using Smartphone-Based Accelerometer Data to Objectively Assess Outcomes in Spine Surgery. Neurosurgery 2021;88:763-72. [Crossref] [PubMed]
- Ford JJ, Kaddour O, Page P, et al. A multivariate prognostic model for pain and activity limitation in people undergoing lumbar discectomy. Br J Neurosurg 2020;34:381-7. [Crossref] [PubMed]
- Rundell SD, Pennings JS, Nian H, et al. Adding 3-month patient data improves prognostic models of 12-month disability, pain, and satisfaction after specific lumbar spine surgical procedures: development and validation of a prediction model. Spine J 2020;20:600-13. [Crossref] [PubMed]
- Gilmore SJ, Hahne AJ, Davidson M, et al. Predictors of substantial improvement in physical function six months after lumbar surgery: is early post-operative walking important? A prospective cohort study. BMC Musculoskelet Disord 2019;20:418. [Crossref] [PubMed]
- Bono OJ, Poorman GW, Foster N, et al. Body mass index predicts risk of complications in lumbar spine surgery based on surgical invasiveness. Spine J 2018;18:1204-10. [Crossref] [PubMed]
- Zheng F, Sandhu HS, Cammisa FP Jr, et al. Predictors of functional outcome in elderly patients undergoing posterior lumbar spine surgery. J Spinal Disord 2001;14:518-21. [Crossref] [PubMed]
- Catalyst N. What is value-based healthcare? NEJM Catalyst 2017. Available online: https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0558#


