
Giant cell tumor of the thoracic spine causing acute paraplegia—a case report
Introduction
Giant cell tumors (GCTs) are rare and account for approximately 5% of all primary bone tumors (1-3). These are typically single tumors located in the metaphysis or meta-epiphysis of long bones in skeletally-mature adults aged 30–40 years old (3,4). Specifically, GCTs in the spine make up less than 10% of all GCTs, and less than 5% percent of all primary spinal tumors (1,5,6). Most commonly, GCTs of the spine occur in the sacrum (5,7). Less than 10% of spinal GCT occur above the sacrum and cases involving the thoracic spine are seldom reported in the literature (2,3,5,8,9).
Although these tumors are most commonly benign, they are often locally invasive and can compromise adjacent tissue. GCTs of the spine often expand into the spinal canal and cause compression of the spinal cord and surrounding vasculature. The most common presenting symptoms of spinal GCTs include back pain and varying levels of neurological dysfunction corresponding to the level of the spinal cord at which they occur and the extent of spinal cord and nerve root involvement (5,7). These general symptoms of back pain and neurologic dysfunction have multiple etiologies that are much more commonly reported than GCTs of the spine, therefore, the diagnosis of GCTs of the spine may be delayed due to the rare nature of these tumors (3,5,7). Given the rarity and complexity of these tumors in the thoracic spine, we present a case of a GCTs occurring in a young female who experienced rapid neurological compromise in the setting of GCTs, followed by remarkable and timely recovery with surgical intervention. The following case presented is in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-652).
Case presentation
A 21-year-old African-American female presents to the emergency department on 05/04/2015 with a back injury while attempting to lift a patient at work by herself. She felt a ‘pop’ and thought she strained her back. Her legs became weak and she developed paresthesias to bilateral lower extremities. On presentation, she reported constant, throbbing, 7/10 pain radiating up and down her spine. At that time, she had no objective motor or sensory deficits. She denied loss of bladder or bowel control with progressive mid-thoracic back pain. She described two months of progressively worsening mid-thoracic back pain radiating to her neck, which was recalcitrant to over-the-counter pain medication. Her past medical history consisted of asthma and she denied any previous surgical history. After a negative workup, she was discharged home with methocarbamol and ibuprofen and told to follow-up with her primary care physician.
After discharge, her back pain worsened. When she woke up the next morning, she was unable to move or feel her bilateral lower extremities and returned to the emergency department. On exam, she had no motor or sensory function below the T8 level. An MRI and CT scan demonstrated near complete destruction of T8 vertebral body with a possible pathologic lesion and fracture associated with dorsal and ventral spinal cord compression and significant signal change within the spinal cord (Figures 1,2). An epidural collection was identified compressing dorsal aspect of T5 to T7.
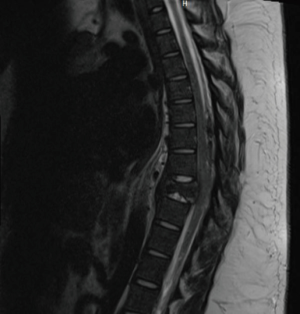
The patient was taken to the operating room the next day for a T7-T9 laminectomy and excisional biopsy of the epidural mass. An initial laminectomy at T8 revealed a large epidural mass extending proximally consistent with a hematoma. Frozen and permanent specimens of the soft tissue mass as well as deep tissue cultures were sent to pathology, which revealed spindle cells, giant cells, and fracture callus. A diagnosis of GCT of the thoracic spine was made.
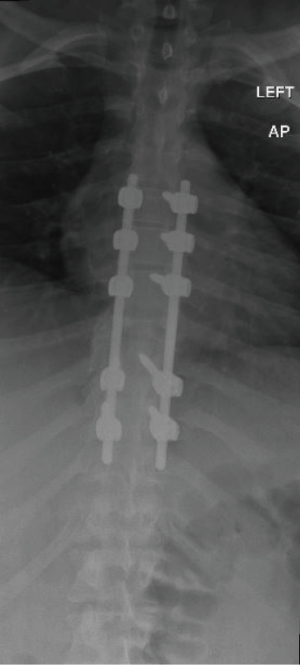
Over the next few days, the patient gained the ability to dorsiflex and plantarflex her toes and slightly flex her hips and knees. Sensation was also improved to her lower extremities. Due to the large mass at T8 and now additional instability because of the fracture anteriorly and wide decompression posteriorly, she was taken for additional surgical stabilization on 5/8/2015. A posterior T5 to T10 instrumented fusion was performed (Figure 3). The patient was discharged to rehab on 5/22/2015.
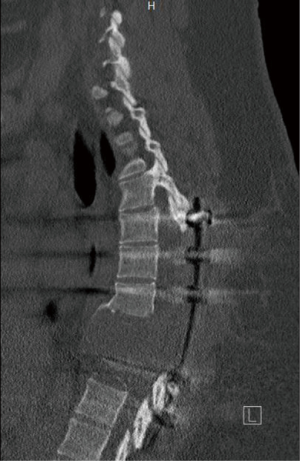
Over the next several months, her leg strength continued to improve, allowing her to ambulate with a rolling walker. On eight-month follow-up, a CT scan demonstrated a lytic lesion at T8 with sclerotic margins. At 14 months follow-up, she reported paresthesias in both legs and had positive Babinski reflex. CT scans at this time demonstrated recurrence of her tumor extending down from T7 to T9 with significant destruction of the vertebral bodies (Figure 4). Due to recurrence of tumor causing severe stenosis and instability as well as spinal cord compression, additional debridement of the tumor and restabilization of her spine was indicated. She was taken to the operating room on 8/2/2016 for a two-stage procedure including hardware removal and extension of posterolateral fusion from T4-T12 followed by an anterior thoracotomy, multiple-level corpectomy, and wide excision of anterior tumor (Figures 5,6).
At three-month follow-up, she ambulates with a rolling walker and numbness and tingling continued to improve. At this time, she began 120 mg denosumab injections every other month. At one-year follow-up, she was able to ambulate without assistive devices and had 5/5 muscle strength throughout. CT scan demonstrated intact hardware with a solid fusion mass around the cage at the T7-T9 corpectomy site with no evidence of recurrent tumor. At four-year follow-up, she continues to live an active lifestyle. She will continue with CT scans every six months to evaluate for tumor recurrence. A timeline has been provided for clarification of sequence of events (Figure 7).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
GCTs are a rare, locally aggressive benign primary bone neoplasms with an estimated incidence of 1.3 per million per year (10,11). They are slightly more common in women than men, with the peak incidence between ages 30 and 40 years.
The most common presenting symptom for spinal GCTs is pain. This usually is compounded by radiculopathy (30%) or paraparesis (16%) due to nerve root or spinal cord compression (12,13). In this case, back pain and paraplegia developed one day following initial presentation, suggesting a more acute presentation than previously discussed in the literature. Many studies have demonstrated the slow progression and invasion of GCTs of the spine. To our knowledge, this case is the first to report an acute progression of paraplegia secondary to a GCT of the thoracic spine.
The preferred treatment for spinal GCTs is complete surgical resection to reduce risks of neurologic damage and tumor recurrence. In cases of spinal GCTs presenting with limited surgical access or in patients who are deemed to have a high-risk of postoperative morbidity, complete resection is often not possible. Some alternative treatments have been reported in the literature with varying levels of success including preoperative embolization with intralesional resection and adjuvant radiotherapy (2,5,7,14). There is currently no standard protocol for treatment of these patients and there is a lack of evidence within the current literature regarding the efficacy of alternative treatments for these tumors (2,5,14). The high recurrence rates following incomplete resection of GCTs of the spine remains a concern (14).
Multiple studies have demonstrated the efficacy of denosumab for the treatment of GCTs (15,16). One study in particular demonstrated no disease progression in 96% of patients after a median follow-up time of 13 months (15). The duration of denosumab treatment postoperatively is controversial. Xu et al. reviewed 102 patients who underwent total en block spondylectomy and reported that postoperative bisphosphonate treatment significantly reduced tumor recurrence rate (17). Conversely, >50% of patients not treated with postoperative bisphosphonate therapy, experienced recurrence of GCTs within a mean follow-up period of 39.9 months.
Based on this evidence, we recommend that denosumab be utilized prophylactically after tumor resection. Additionally, we suggest denosumab be discontinued six months after surgery and long-term follow-up spinal radiographs and CTs to evaluate for potential tumor recurrence. In this case, the patient will be reevaluated for tumor recurrence every six months with radiographs and CT scans. This paper reports the case of a young female presenting with a GCT of the T8 vertebra with associated back pain and paraplegia. Following complete surgical excision and a course of denosumab, symptoms were resolved and there has been no recurrence of the GCTs to date.
This is an isolated case of GCT of the thoracic spine with only 4 years of follow-up. Due to the risk of recurrence of the tumor, a longer period of follow-up would be ideal. This case adds to the existing literature of presentation and management of GCT of the thoracic spine, a rare presentation of this pathology. The unique presentation of this patient is a strength of this case report as it provides novel information on the diagnosis and management of patients with GCT of the spine who present in this manner. Additionally, the successful management of this patient’s tumor recurrence with surgical excision and denosumab supports previous reports of the treatment of recurrent GCT’s of the spine and adds to the available body of literature on this topic.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-652
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-652). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ben Nsir A, Said IB, Badri M, et al. Giant cell tumor of the sixth thoracic vertebra: case report. Turk Neurosurg 2015;25:475-8. [PubMed]
- Kim HS, Lee JE, Jung SS, et al. Spinal Cord Injury due to the Giant Cell Tumor of the Second Thoracic Vertebra: A Case Report. Ann Rehabil Med 2013;37:269-73. [Crossref] [PubMed]
- Palmerini E, Picci P, Reichardt P, et al. Malignancy in Giant Cell Tumor of Bone: A Review of the Literature. Technol Cancer Res Treat 2019;18:1533033819840000 [Crossref] [PubMed]
- Yu H, Shi R, Peng ZG, et al. Primary malignancy in giant cell tumor of thoracic vertebrae: A case report. Medicine (Baltimore) 2018;97:e11484 [Crossref] [PubMed]
- Al-Shamary E, Al-Dhafeeri W, Al-Sharydah A, et al. Total Spondylectomy for Upper Thoracic Spine Giant Cell Tumor: A Case Report. Case Rep Oncol 2019;12:131-8. [Crossref] [PubMed]
- Zheng K, Xu M, Wang B, et al. Giant Cell Tumor of the Mobile Spine Occurring in Pregnancy: A Case Report and Literature Review. Orthop Surg 2017;9:252-6. [Crossref] [PubMed]
- Martin C, McCarthy EF. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J 2010;30:69-75. [PubMed]
- Kelly D, Mc Erlean S, Byrne D, et al. A case of thoracic giant cell tumor of bone and discussion of radiological features and current management practices. Radiol Case Rep 2016;11:222-6. [Crossref] [PubMed]
- Lee CG, Kim SH, Kim DM, et al. Giant cell tumor of upper thoracic spine. J Korean Neurosurg Soc 2014;55:167-9. [Crossref] [PubMed]
- Rockberg J, Bach BA, Amelio J, et al. Incidence Trends in the Diagnosis of Giant Cell Tumor of Bone in Sweden Since 1958. J Bone Joint Surg Am 2015;97:1756-66. [Crossref] [PubMed]
- Balke M, Henrichs MP, Gosheger G, et al. Giant cell tumors of the axial skeleton. Sarcoma 2012;2012:410973 [Crossref] [PubMed]
- Deveci MA, Paydaş S, Gönlüşen G, et al. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: Prospective study of 14 cases. Acta Orthop Traumatol Turc 2017;51:1-6. [Crossref] [PubMed]
- Inoue G, Imura T, Miyagi M, et al. Total en bloc spondylectomy of the eleventh thoracic vertebra following denosumab therapy for the treatment of a giant cell tumor. Oncol Lett 2017;14:4005-10. [Crossref] [PubMed]
- Kim SC, Cho W, Chang UK, et al. Clinical Outcome of Treatment for Patients with Giant Cell Tumor in Spine. J Korean Neurosurg Soc 2015;58:248-53. [Crossref] [PubMed]
- Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013;14:901-8. [Crossref] [PubMed]
- Chawla S, Blay JY, Rutkowski P, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol 2019;20:1719-29. [Crossref] [PubMed]
- Xu W, Li X, Huang W, et al. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol 2013;20:804-10. [Crossref] [PubMed]






