
Postoperative spinal epidural hematoma following therapeutic anticoagulation: case report and review of literature
Introduction
Spinal epidural hematoma requiring surgical evacuation is a rare but severe complication of spine tumor surgery with an incidence of 0.30% (1). Previous studies have shown that patients undergoing major elective orthopaedic surgery have a significant risk of developing deep vein thrombosis (DVT), which may require intervention to prevent a fatal pulmonary embolism (PE) (2-4). In addition, the cardiac stress produced by the general anesthesia required for major spine surgery can cause myocardial ischemia and/or infarction, thus necessitating perioperative anticoagulation (5).
However, prophylactic anticoagulation is controversial and many surgeons may instead prefer mechanical prophylaxis to avoid the morbidity and bleeding risk associated with anticoagulation after spine surgery (6). In established cases of PE that may be potentially life threating following spinal surgery, therapeutic anticoagulation with adjusted dose heparin or a low molecular weight heparin (LMWH) may be required to prevent further propagation of the clot. However, this must be balanced with the risk of complications including spinal hematoma, wound breakdown and cord compression (7).
Here we present a rare case of spinal epidural hematoma following the administration of therapeutic clexane (enoxaparin) after spinal surgery and review the current guidelines on the initiation of therapeutic anticoagulation after spine surgery. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-636).
Case presentation
A 56-year-old man with a medical history of renal calculi was noted to have an incidental (4 cm × 5 cm) left renal mass on computer tomography (CT) scan, but defaulted follow-up on the lesion for 7 months. He presented with 5 weeks of progressive bilateral lower limb weakness, sensory loss and incontinence. He was unable to walk, and was wheelchair bound. On admission, the power of his lower limbs was weak, (Medical Research Council, grade 3 power for L2 and L3, grade 4 for L4–S1). Sensation was also reduced in the L2 dermatome and the anal sphincter tone was lax.
Urology review indicated a high suspicion for renal cell carcinoma (RCC) with likely metastases to the spine and causing the left renal vein thrombus. He was started on dexamethasone and scheduled for an inferior vena cava (IVC) filter insertion for embolus prevention.
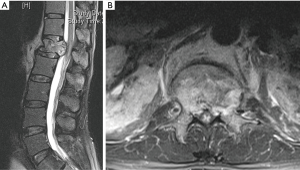
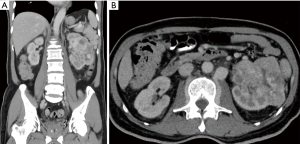
On day 2 of admission, his neurologic status worsened (MRC, grade 1 power for L2 and L3, grade 2 power for L4–S1). Blood investigation showed mildly elevated inflammatory markers (white blood cell, 8.06; erythrocyte sedimentation rate, 52; C-reactive protein, 71.4), low prostate specific antigen, 0.79 and normal urine formed elements and liver function. His preoperative hemoglobin was 11.2 with normal coagulation profiles [activated partial thromboplastin time (APTT) 29.3, partial thromboplastin time (PTT) 13.0 and international normalised ratio (INR) 1.2]. Magnetic resonance imaging (MRI) revealed an acute compression fracture of the L1 vertebral body with compressive myelopathy of conus medullaris (Figure 1). CT scan showed a left renal carcinoma (9.4 cm × 8.1 cm) with filling defect in left renal vein and IVC (Figure 2).
The patient underwent a complete L1 laminectomy, partial T12 laminectomy, T11–L3 stabilization and posterior lateral and inter-facet fusion. The surgery was uneventful with 500 mL of blood loss, and there were no complications or dural tear intraoperatively.
Between postoperative day (POD) 1–6, the patient showed marked improvement in lower limb neurology. He was able to ambulate with a walking frame and with minimal assistance. Anal sphincter control had also return. His surgical wound was dry, and the surgical drain was removed without issues on POD 4. He was kept on mechanical thromboprophylaxis daily following his operation.
In view of persistent left renal vein thrombus, therapeutic subcutaneous clexane was initiated at 80 mg (1 mg/kg) twice daily, as per hematology, due to the following indications: (I) prothrombotic state from metastatic disease, (II) prevention of thrombus propagation, and (III) prevention of IVC filter blockage. Clexane was started on the night of POD 6 and he was subsequently discharged on the night of POD 7.
In the evening of POD 8, the patient was readmitted with progressive paralysis of his lower limbs. Clinical examination revealed motor power of grade 1 from L2–L3 and grade 0 from L4–S1 with parasthesia over the left L3–L5 dermatome. Bilateral plantars were upgoing. There was perianal anaesthesia and lax anal tone.
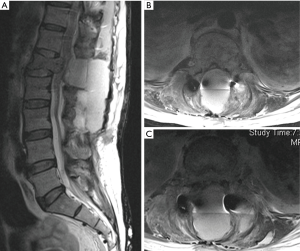
MRI scan revealed an interval development of a large posterior epidural and subcutaneous hematoma, with severe compression of the conus at L1 level (Figure 3). X-ray of thoracolumbar spine showed no periprosthetic loosening and IVC filter being in situ.
Urgent posterior decompression of hematoma and exploration was performed on the night of admission. Intravenous protamine (50 mg) was given to reverse effects of clexane. Intraoperatively, a large haematoma was noted around the previous surgical site with generalised bleeding and 1.5 L of blood loss. Postoperatively, patient was kept on mechanical thromboprophylaxis strictly.
The recovery of the patient’s neurology following the second operation took a significantly longer duration. His power improved gradually over the right lower limb with attainment of grade 4/5 motor power but still had hemiparesis on his left lower limb upon discharge out of hospital.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Surgical Site Hematomas is a known complication especially in patients who are already on antiplatelet or anticoagulation therapy. For this, clear guidelines exist to help the clinician reduce this complication. However, there is a paucity of literature regarding when to initiate therapeutic anticoagulation for high risk patients in the early post-operative period to prevent the risk of a fatal PE (5,8,9). This case report highlights the possible complication of postoperative spinal epidural hematoma associated with the initiation of therapeutic clexane (enoxaparin, a LMWH) after spine surgery.
Decortication of the spine and the large potential dead space created during exposure predisposes to haemorrhagic complications and hematoma formation after spine surgery (10). While most postoperative spinal epidural hematomas are clinically asymptomatic, the rare hematoma that causes significant spinal cord compression can result in devastating neurologic consequences—commonly bilateral lower limb paraparesis, paraplegia or cauda equina syndrome (11,12).
The management of thromboembolic complications is difficult in spine surgery patients because of the bleeding risks associated with initiating anticoagulation (4,13). Several questions remain unanswered regarding the guidelines of postoperative anticoagulation in spine surgery patients.
Should anticoagulation be started prophylactically for spine surgery patients?
Low risk patients
The risk of spinal epidural hematoma associated with heparinization has led many authors to advocate against pharmacological prophylaxis for routine spine surgery, particularly after decompressive laminectomy (6). According to the North American Spine Society’s (NASS) Evidence-based clinical guidelines for Antithrombotic Therapies in Spine Surgery (9), most commonly performed elective spine surgeries only carry a very low risk of venous thromboembolism (VTE) and thus chemoprophylaxis may not be necessary in such cases.
High risk patients
According to the American College of Chest Physicians (ACCP) Recommendations for Spine Surgery (8), mechanical and chemoprophylaxis (Heparin or LMWH) have been recommended for patients undergoing spine surgery, especially in those with additional risk factors such as circumferential spine surgery, multiple trauma, malignancy or hypercoagulable states. In a multicentre, randomized, controlled trial by Agnelli et al. (14), the authors found that enoxaparin with compression stockings was more effective than compression stockings alone for the prevention of DVT after elective neurosurgery, and did not cause excessive bleeding. Hence, while low-risk patients do not require chemoprophylaxis, high-risk patients should be started on both mechanical and chemoprophylaxis to avoid the devastating consequence of a PE.
What is the dosing regimen of therapeutic anticoagulation that should be started after spine surgery?
For patients with established thromboembolic disease (e.g., DVT, PE), therapeutic anticoagulation should be initiated. Therapeutic doses of enoxaparin are typically 1 mg/kg twice daily (as in the present case study), while the prophylactic dose is 40 mg, once daily (15). In contrast, therapeutic heparin is initiated as an intravenous loading dose followed by a 1,000 units/h infusion (16). Additionally, heparin requires close monitoring and titration of the infusion to ensure a therapeutic range of 0.3–0.7 U/mL by anti-Xa analysis (17).
More recently, LMWH (Enoxaprin) has been preferred over heparin because of its ease of use in attaining therapeutic level clinically and not requiring an infusion (18). Shiu et al. (19) conducted one of the only studies to evaluate outcomes of spine surgery patients undergoing therapeutic anticoagulation for thromboembolic disease. Compared to patients treated with LMWH, those treated with heparin infusion had overall higher reoperation rates due to bleeding-associated complications. The authors also found that patients with complications in the heparin group had more supratherapeutic PTT measurements compared to those without complications. Hence, the higher bleeding risk associated with heparin could be, in part, due to the greater difficulty in achieving optimal levels clinically and that it might be safer to use therapeutic LMWH to treat spine surgery patients who develop postoperative VTE. Nonetheless, it is important for clinicians to remember that LMWH still carries a risk of bleeding complications like spinal epidural hematoma, as in this case.
When should anticoagulation be started after spine surgery?
In reviewing the available literature, several factors need to be considered before initiating anticoagulation, including ensuring the wound has completely healed, a low drainage output and timing of drain removal when a drain is used, the underlying pathological condition, comorbidities, and other host factors, such as ambulatory and neurological status of each patient (9). A recent study by De la Garza Ramos et al. (20) found that administration of prophylactic anticoagulation between POD 1-3 for metastatic tumors of the spine significantly reduced the risk of VTE than administration on or after POD 4. However, it is important to balance the benefits of early administration of prophylactic anticoagulation with the associated risks of bleeding complications.
Cain et al. (7) performed one of the only other studies investigating therapeutic heparin use in patients undergoing spine surgery after PE. Intravenous heparin was started at the time of diagnosis of PE, ranging from POD 1 to 14 among the 9 patients who were included in the study. Bleeding complications occurred in 6 of the 9 patients from POD 1 to 9 but not in the remaining patients when therapeutic heparin was initiated from POD 12 to 14 (7). Likewise, in the present case study, the patient developed a spinal epidural hematoma on POD 8 after initiation of therapeutic clexane on POD 6. The existing data suggest that it might be safer to initiate postoperative therapeutic anticoagulation no earlier than from POD 10 to 14 to reduce the risk of bleeding complications.
What is the role of IVC filters as an alternative to anticoagulation after spine surgery?
The morbidity of IVC filter placement is low and should be considered as a management alternative in the treatment of patients who are at a high risk of PE after surgery (7). Previous studies (21,22) have shown that prophylactic IVC filter placement in high-risk spine surgery patients significantly reduced the odds of developing a PE as compared to control populations.
Hence, given the efficacy of IVC filters in preventing adverse complications of PE, such measures should be considered as an alternative to anticoagulation, especially in patients with an established DVT who require surgery. The additional morbidity and risks of hemorrhagic complications of anticoagulation also further account for why spine surgeons, advocate IVC filters for definitive treatment of PE in the postoperative period of spinal surgery (7).
In conclusion we have presented a case of postoperative spinal epidural hematoma associated with the initiation of therapeutic clexane after spine surgery. When initiating anticoagulation in patients who are at high risk of PE postoperatively, the clinician must balance the risk of bleeding complications (such as spinal epidural hematomas) with the devastating risk of a PE. In such cases, therapeutic anticoagulation should still be initiated as indicated, after carefully considering the choice of drug, dosage, timing and use of an IVC filter. Clinicians should subsequently be wary and monitor for the rare bleeding complications associated with such therapy so that they can be identified and addressed early, should they occur.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-636
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-636
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-636). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gao X, Li L, Cao J, et al. Symptomatic postoperative spinal epidural hematoma after spine tumor surgery: Incidence, clinical features, and risk factors. Spinal Cord 2019;57:708-13. [Crossref] [PubMed]
- Kou J, Fischgrund J, Biddinger A, et al. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976) 2002;27:1670-3. [Crossref] [PubMed]
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:338S-400S. [Crossref] [PubMed]
- Schulte LM, O'Brien JR, Bean MC, et al. Deep vein thrombosis and pulmonary embolism after spine surgery: incidence and patient risk factors. Am J Orthop (Belle Mead NJ) 2013;42:267-70. [PubMed]
- Barnes B, Alexander JT, Branch CL Jr. Postoperative Level 1 anticoagulation therapy and spinal surgery: practical guidelines for management. Neurosurg Focus 2004;17:E5. [Crossref] [PubMed]
- Epstein NE. A review of the risks and benefits of differing prophylaxis regimens for the treatment of deep venous thrombosis and pulmonary embolism in neurosurgery. Surg Neurol 2005;64:295-301; discussion 302. [Crossref] [PubMed]
- Cain JE Jr, Major MR, Lauerman WC, et al. The morbidity of heparin therapy after development of pulmonary embolus in patients undergoing thoracolumbar or lumbar spinal fusion. Spine (Phila Pa 1976) 1995;20:1600-3. [Crossref] [PubMed]
- Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:7S-47S.
- Bono CM, Watters WC 3rd, Heggeness MH, et al. An evidence-based clinical guideline for the use of antithrombotic therapies in spine surgery. Spine J 2009;9:1046-51. [Crossref] [PubMed]
- Yi S, Yoon DH, Kim KN, et al. Postoperative spinal epidural hematoma: risk factor and clinical outcome. Yonsei Med J 2006;47:326-32. [Crossref] [PubMed]
- Park JH, Park S, Choi SA. Incidence and risk factors of spinal epidural hemorrhage after spine surgery: a cross-sectional retrospective analysis of a national database. BMC Musculoskelet Disord 2020;21:324. [Crossref] [PubMed]
- Masuda S, Fujibayashi S, Takemoto M, et al. Incidence and Clinical Features of Postoperative Symptomatic Hematoma after Spine Surgery: A Multicenter Study of 45 Patients. Spine Surg Relat Res 2020;4:130-4. [Crossref] [PubMed]
- Morse K, Weight M, Molinari R. Extensive postoperative epidural hematoma after full anticoagulation: case report and review of the literature. J Spinal Cord Med 2007;30:282-7. [Crossref] [PubMed]
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998;339:80-5. [Crossref] [PubMed]
- Horlocker TT, Vandermeuelen E, Kopp SL, et al. Regional Anesthesia in the Patient Receiving Antithrombotic or Thrombolytic Therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med 2018;43:263-309.
- Spanier DE, Stambough JL. Delayed postoperative epidural hematoma formation after heparinization in lumbar spinal surgery. J Spinal Disord 2000;13:46-9. [Crossref] [PubMed]
- Byun JH, Jang IS, Kim JW, et al. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res 2016;51:171-4. [Crossref] [PubMed]
- Hylek EM, Regan S, Henault LE, et al. Challenges to the effective use of unfractionated heparin in the hospitalized management of acute thrombosis. Arch Intern Med 2003;163:621-7. [Crossref] [PubMed]
- Shiu B, Le E, Jazini E, et al. Postoperative Deep Vein Thrombosis, Pulmonary Embolism, and Myocardial Infarction: Complications After Therapeutic Anticoagulation in the Patient With Spine Trauma. Spine (Phila Pa 1976) 2018;43:E766-72. [Crossref] [PubMed]
- De la Garza Ramos R, Longo M, Gelfand Y, et al. Timing of Prophylactic Anticoagulation and Its Effect on Thromboembolic Events After Surgery for Metastatic Tumors of the Spine. Spine (Phila Pa 1976) 2019;44:E650-5. [Crossref] [PubMed]
- McClendon J Jr, O'Shaughnessy BA, Smith TR, et al. Comprehensive assessment of prophylactic preoperative inferior vena cava filters for major spinal reconstruction in adults. Spine (Phila Pa 1976) 2012;37:1122-9. [Crossref] [PubMed]
- Rosner MK, Kuklo TR, Tawk R, et al. Prophylactic placement of an inferior vena cava filter in high-risk patients undergoing spinal reconstruction. Neurosurg Focus 2004;17:E6. [Crossref] [PubMed]


