
Implications for diagnosis and treatment of peri-spinal implant infections from experiences in periprosthetic joint infections—a literature comparison and review
Introduction and methods
Periprosthetic joint infections (PJI) are among the most devastating complications possible in arthroplasty (1). Both the economic impact (2,3) and the complexity in treatment (4) have made PJI one of the most important topics for orthopedic research in the last decade. The most thoroughly investigated entities include the hip and knee joint (5,6), however, also shoulder and elbow arthroplasty came into the focus in recent years’ research (7,8). Compared with arthroplasty infections, peri-spinal implant infections (PSII) pose a similar problem with similar diagnostic and therapeutical approaches (9). However, existing research in the field is limited compared to PJI. With our review we aim to evaluate existing similarities and differences between PJI and PSII and to develop relevant implications for diagnosis and treatment of PSII based on literature and knowledge about PJI.
This review was performed on the basis of a PubMed, Cochrane Library and Medline literature analysis. In addition, literature was considered that was only identified in the references part of other studies and not in the primary literature analysis itself. No approval by an ethics committee was necessary and no conflict of interest was present. The key search terms included “periprosthetic joint infection (PJI)”, “perispinal implant infection (PSII)”, “spinal implant infection”, “arthroplasty infection”, “spine infection” and “spine implant infection”. For a better overview, this review will primarily focus on the comparison of hip and knee arthroplasty with spine instrumentation. If not stated otherwise results and studies mentioned in the review reporting PJI thus refer to hip and knee arthroplasty.
Results
At the time of the finalization of the literature search for this review (12/2019), a PubMed search using the term “periprosthetic joint infection” resulted in 2,636, the term “spinal implant infection” in 734 results, the Cochrane Library search yielded 118 results for “periprosthetic joint infection”, and 107 for “spinal implant infection”. Overall, 99 references were included in this review, of which 63 were original studies, 27 reviews (including 7 meta-analyses) and 9 expert, consensus and international guideline references.
Definition
In the current literature, a PJI is usually either defined using the Musculoskeletal Infection Society (MSIS) criteria (10), the European Bone and Joint Infection Society (EBJIS) criteria (11) or the Infectious Diseases Society of America (IDSA) (12) criteria. All three definitions have in common that microbe detection is not ultimately necessary for the definition of a PJI. Other criteria like histopathology, leucocyte count from synovial fluid and clinical signs like a sinus tract, known to possess high sensitivity and specificity for a PJI, are considered to be sufficient as well for the diagnosis, either isolated or combined. Table 1 is giving an overview over the different PJI definitions.

Full table
A re-infection following an initial PJI treatment can be defined via the Delphi Consensus criteria which include a further subsequent revision for PJI, death by PJI and a postoperative wound healing delay as defining criteria (13). In contrast to that, up to this point no specific definition for PSII has been established. At the moment only general definitions for spinal surgical site infections are present, differentiating between superficial and deep infection (14). However, a time onset of 3-month after an operation is a possible definition criterion for a delayed infection in both PJI and PSII (1,9).
Incidence
The frequency of PJI primarily depends on the involved joint. While in hip and knee joint the incidence is about 1–2% (2), the incidence in shoulder arthroplasty has been reported to be only 1% (7), and up to 3% in elbow arthroplasties (8). The reported incidence rates of PSII vary within a much broader spectrum without any existing meta-analysis and relatively small patient samples. While some studies describe rates of infection between 1% and 4% for instrumented spine surgery (15), some reviews state rates of up to 20% (9). In both, PJI and PSII, the exact number is depending on the type of study, the subpopulation, the exact type of surgery and several further factors. In both PSII (16) and PJI (2) the absolute numbers are expected to rise in the future following an elderly western population and increasing numbers of both arthroplasties and spinal fusions.
Risk factors
Risk factors for both PJI and PSII are similar, especially when considering general patient condition factors such as age, obesity, diabetes, rheumatoid arthritis, smoking, immunosuppression, malignancy, and prior revisions (9,17-20). Male gender was identified as an additional risk factor for both PJI and PSII in some studies (1,9).
Similar to PJI, infections following spinal surgery are associated with prolongated length in operation time and subsequently increased blood loss. Surgical risk factors for PSII and PJI, such as operative approach and implanted material, do differ between the two entities. In PSII, a posterior approach in spinal instrumentation is associated with a higher, an anterior instrumentation with a lower risk of infection (21). One study with less than 100 patients showed that titanium implants were associated with a lower rate of infection compared to stainless steel (22), while one animal study demonstrated that polyetheretherketone polymer was associated with a higher rate of infection compared to titanium and silicon nitride (23). However, the available literature concerning the outcome of different materials used in spinal surgeries remains limited when considering the number of patients and the study setting. In contrast, in arthroplasty, Lenguerrand et al. were able to show that the use of ceramic was associated with a decreased risk of PJI following primary arthroplasty when compared to metal bearings, using a national wide data based prospective observational cohort study with 2,705 PJI cases (24). A further study, using Medicare data [2005–2009], by Bozic et al. identified metal-on-metal bearings as a risk factor for PJI compared to metal-on-polyethylene bearings and ceramic-on-ceramic bearings analyzing 148,827 cases (25). One study with over 4,000 arthroplasty surgeries in knee, hip and elbow joints identified an increased PJI risk with metal-to-metal hinged knee prosthesis compared to metal-polyethylen (26). However, the study included both revision (primarily metal-to-metal hinged) and primary arthroplasty (primarily metal-polyethylen) cases developing a PJI, which makes the results hard to interpret due to the skewed groups. Latest in vitro results indicate that a titanium implant coating might be a protective factor against PJI (27).
Pathogenesis
When comparing the microbes involved in PSII and PJI, the main problems include the fact that the number of cases in studies describing perispinal implant infections is limited when compared to PJI, and that the term post-operative spinal wound infection is used without differentiating between implant associated infections like instrumentation and non-implant associated infections like the ones following a discectomy. In this review, the Mayo Clinic Prosthetic Joint Infection Database (E. F. Berbari) (1) as one of the largest PJI data sets was compared with three of the most frequently cited studies dealing with spinal implant infections (Table 2). Staphylococcus aureus and Coagulase negative Staphylococci dominate the microbe spectrum in both PJI and PSII. This result is also backed by further studies investigating PSII (31-33). However, when compared to PJI, postoperative spinal infections demonstrate a more polymicrobial spectrum, with aerobic gram-negative bacteria like E. coli on the one hand, and the Enterococcus species group on the other hand, as additional dominating infection groups. In both, PJI and PSII, biofilm formation on the implants by the causative organisms is the major diagnostic and therapeutic problem. This is the case, on the one hand, due to increased tolerance against antibiotics of bacteria in biofilm and, on the other hand, due to the reduced ability to successfully aspirate the microbe in the course of the initial diagnosis. Both problems are caused by the biofilm formation surrounding the prosthesis and subsequently protecting and covering the microbe (34,35).
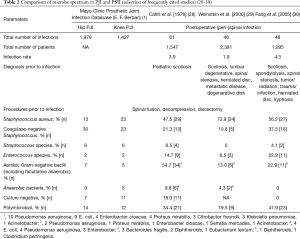
Full table
Diagnosis
In both PJI and PSII an algorithm-based diagnosis approach has been proposed (36,37). The algorithm-based models include the diagnostical combination of symptoms, clinical examination, fast screen lab values like CRP, aspiration (cellular composition of synovial and periimplant fluid, microbe identification), intraoperative tissue samples, histopathology, and imaging like X-ray or additional MRI and/or CT scans. The clinical symptoms of PJI and PSII are similar and include local pain, swelling and redness. Low-grade microbes primarily cause chronic infections, high-grade ones a more acute symptom onset. A further differentiation, especially used in spinal infections, is based on the involved tissue layer as superficial compared to deep infection. In addition to that, a differentiation is possible based on the suspected focus as local, hematogenous and per continuitatem (1,9). Both the anamnesis for a possible PJI and PSII should include the patient’s prior conditions and operations, as well as the current course of the symptoms. The clinical examination should include the local status and the search for possible hematogenous foci such as infections of teeth or feet (38,39).
Following the initial anamnesis for patient history, clinical signs and body examination, lab results like an elevated CRP (C-reactive protein), the ESR (erythrocyte sedimentation rate) and the blood leukocyte count are used as first fast screening parameters. The advantages of CRP and ESR were first described in total in the context of TKA (Total Knee Arthroplasty) by Austin et al. in 2008 as “cost-effective, highly sensitive, and low risk to patients”. However, the combined use of CRP and ESR in the study only demonstrated a high negative predictive value, for the cost of a relatively low positive predictive value (40). Berbari et al. published meta-analysis from 2010 included over 30 studies dealing with PJI in both THA (Total Hip Arthroplasty) and TKA. He identified the diagnostic accuracy for PJI to be the highest with interleukin-6, followed by CRP, ESR, and white blood-cell count (41). The same study also demonstrated that lab values such as CRP are problematic in the case of chronic low-grade infections given their relatively low sensitivity and specificity. Despite its downsides, the determination of both CRP and ESR in suspected PJI, has become a standardized part of diagnosis of PJI and is also part of the latest AAOS (American Academy of Orthopaedic Surgeons) recommendations (42). In contrast, data concerning the usage of fast screening parameters for the diagnosis of PSII are limited. Similar to the diagnosis of PJI, the detection of low-grade spinal implant infections using CRP and ESR remains problematic. A 10-year retrospective study from Oxford University published in 2008 demonstrated that “17% of CRP results, 45% of ESR and 95% of WBC results were within the normal range prior to the diagnosis of infection” (43). However, the study analyzed only 74 patients, of whom the low-grade microbe Propionibacterium was identified in 34 intraoperative tissues. A further retrospective study by Akgün et al. even warned that the use of only CRP misdiagnoses low-grade PSII. In the study 43% of the PSII group had a CRP <5 mg/L prior to revision surgery (sensitivity 64%, specificity 68%) (44). In contrast to that, Dobran et al.’s retrospective study, that included a PSII and a healthy control group, identified ESR and CRP as the only statistically significant parameters, while compared to that, fever, number of leukocytes, neutrophils and lymphocytes were not statistically significant for an infection (45).
Joint aspiration in cases of PJI is a well-established procedure offering the opportunity to analyze white blood cell (WBC) count, differential count and culture in one procedure (46). Thereby, synovial fluid WBC has been demonstrated to have high specificity and sensitivity for PJI, becoming part of both the MSIS and EBJIS definition (10,11). In contrast to that, aspiration cultures show poor sensitivity with negative rates of up to 20% in cases of an actual underlying PJI (37). To our best knowledge, no study has yet systematically described aspiration in the context of PSII. We believe that the limited anatomic options for the procedure might explain this fact. Joint aspiration following an operation should always be considered critical, given the postoperative inflammatory reaction with a physiological rise in WBC, and the subsequent possibility of false positive diagnosis. If used at all, different cutoff values should be adapted, as proposed by Bedair et al. for PJI following primary TKA (27,800 instead of 3,000 cells/µL within the first 6 weeks) (47). In addition, joint aspiration values should always be interpreted together with clinical presentation and blood tests (48).
Following the mentioned initial diagnosis, imaging is oftentimes used as a next step. Thereby, simple X-rays in PJI are only able to demonstrate indirect signs of an infection (early loosening, signs of osteolysis, radiolucency at the cement-bone interface, malrotation) with low sensitivity and specificity (49). Besides, a prior image is necessary for a thorough evaluation (50). However, plain radiographs still might be useful to quickly rule out other causes like fracture (49), with some studies additionally suggesting possible criteria to differentiate between septic and aseptic osteolysis/loosening solely based on plain radiographs (51). Given the low specificity of MRI and CT for PJI, their relative high costs, and the necessary time investment, both are not part of the primary PJI diagnostic algorithm. However, they might play a role in preoperative planning, the diagnosis of possible surrounding defects and of per continuitatem/hematogenous PJI (52). Bone scintigraphy is used relatively seldomly as a diagnostic tool in suspected PJI, and given its high negative predictive value, primarily plays a role as last preoperative option in cases of unclear differentiation between septic and aseptic cases (53). Similar to PJI, diagnostical imaging in cases of suspected PSII involves X-ray, CT, and MRI with important signs including early implant loosening, tissue swelling, loss of height of discs, and implant dislocation. Like imaging in PJI, an acute onset infection is oftentimes not directly present in imaging. Thereby, MRI is showing a higher sensitivity for fluid collections and the exact localization of a possible infection (disc, bone, epidural localization) than X-ray and CT scan, and thus is necessary for both the initial diagnosis and the preoperative planning. While the majority of spine surgeons consider loosening signs to be primarily a consequence of mechanical failure, latest results indicate that low-grade infections, similar to PJI, might be an underrated cause of loosening in spinal surgery (54). Like in PJI, radionuclide imaging is not a primary tool for diagnosis and used as a final preoperative rule out tool (55).
CT guided preoperative biopsies have been described as a commonly used diagnostic procedure in case of suspected spinal infections (56). However, some studies have also evaluated preoperative CT guided aspiration in PJI. One study by Tomas et al. determined a “70% sensitivity, 100% specificity, 84% accuracy, 100% positive predictive value, and 75% negative predictive value” using preoperative CT guided fluid aspiration together with specific CT image findings in 63 patients with clinical suspicion for a hip PJI (57). A similar study by Isern-Kebschull et al. analyzed 96 patients with clinical suspicion for a hip PJI. The combination of CT-guided joint aspiration and CT findings (tissue swelling, prosthesis loosening, osteolysis and ossification, enlarged lymph nodes) enabled an accuracy of 86.5% (58).
Oftentimes however, the definitive and final diagnosis of PJI or PSII is only made intraoperatively via tissue sampling and histopathology. In case of PJI, two samples of the same low-grade microbes or one high grade microbe are necessary for the final diagnosis following the latest EBJIS guidelines (11). In addition to that, histopathological criteria such as the Krenn & Morawietz criteria are used as for standard diagnostics with specificity rates of up to 95% (59). Similar to PJI, in perispinal implant infections the tissue samples can either be gained preoperatively via radiographic guided needle biopsy and/or intraoperatively depending on the initial symptoms. Sign of neurological damage, sepsis and instability require an acute intervention, making a preoperative radiographic guided biopsy less relevant, given the following intraoperative options for tissue sampling. In contrast, less acute settings and unclear clinical situations might justify a preoperative CT guided biopsy (36).
In both PJI and PSII implant sonication has demonstrated promising results in recent years, with studies showing high specificity and reliance with improved diagnostic outcomes. In a prospective controlled consecutive cohort study with more than 100 patients, Bürger et al. were able to show that spinal implant sonication was more sensitive than conventional peri-implant tissue culture for the diagnosis of PSII (60). A further prospective study with more than 100 patients by Sampedro et al. demonstrated similar results with both higher specificity and sensitivity using perispinal implant sonication compared to tissue microbiology (61). However, in both studies the number of actual spinal infections identified in the two patient groups was only 35 and 22 spinal infections, respectively (60,61). A retrospective study by Rothenberg et al. with more than 500 patients demonstrated higher sensitivity using sonication for PJI in arthroplasty compared to synovial fluid culture and tissue culture. However, no difference concerning specificity was identified (62).
Therapy
In both PJI and PSII, the treatment should be ideally performed by an interdisciplinary team of microbiologists, infectiologists, pathologists, radiologists and surgeons in a centralized setting (63,64). A combined surgical and antimicrobial approach is necessary in implant associated infections, rather than an isolated surgical or sole antibiotic procedure.
In PJI, several treatment strategies are used depending on the type of microbe, the onset of symptoms, the local tissue condition and patient status. Debridement with antibiotics and implant retention (DAIR) with the exchange of mobile parts (head and inlay in THA, inlay in TKA) is an established strategy for acute infections, while in chronic infections the entire prosthesis has to be removed and subsequently replaced by a new one. This can be performed in the course of a one-, two-, or multiple stage exchange (64). The main difference between PJI and PSII treatment involves the question whether or not the material should be exchanged. While in PJI only in acute infections a DAIR is recommended to be performed, current studies in PSII tend to prefer a preservation of the infected implant also in chronic cases, given the oftentimes difficult options for implant removal in cases of intervertebral cages and spine vertebral replacements (9,43). The most important exceptions to this rule included absence of wound or bone healing, insufficient wound drainage and new or increasing neurological deficits (9). However, implant preservation in PSII remains controversial with some authors preferring complete material removal for the price of increased risk of spine instability and bone deformation (65,66), while other authors suggest implant removal in PSII only in cases of likely additional loosening (67).
In cases of PSII without the option of implant removal, long term antibiotic suppression therapy might be an option. However, systematic studies evaluating this concept do not exist. In this context possible implications from PJI might be helpful. Here, several studies describe and evaluate indications, options and outcome of long-term suppression therapy (68,69).
In both PJI and PSII the usage of antibiotic loaded bone cement (ALBC) has been an accepted therapy strategy for fixation in the course of reimplantation and as screw fixation. In addition to that, ALBC is used as a spacer following the explanation in PJI (36) or for a reduction of anatomic dead space directly for the treatment of spinal implant infections (70).
Antibiotic therapy in arthroplasty and spinal infections should be based on the latest EUCAST recommendations, the bacterial susceptibility and patient related factors like renal function and bodyweight (9,64). In case of PJI, a combination of intravenous (i.v.) antibiotics directly following the operation and oral antibiotics following the patients discharge is the treatment of choice. Different antibiotic protocols have been published with also variable efficiencies. In our clinics, antibiotics are administered for a total of 10 to 12 weeks without an antibiotic pause period, with 1 to 3 weeks of this time as i.v. antibiotics.
In case of a one-stage exchange or DAIR, two weeks of i.v. antibiotics without antibiofilm activity are initiated and then switched to 10 weeks of p.o. antibiotics ideally with antibiofilm activity. Fourteen days of antibiotics without antibiofilm activity are initially administered due to the risk of rapid resistance development of Staphylococci on the skin against rifampicin (difficult to treat microbe). Within the first 14 days, the risk of postoperative wound healing delay, seroma, and hematoma is present. In case of a prior administration of antibiofilm active AB (rifampicin) combined with a revision within the first 14 days, the spread of Staphylococci from the skin (now potentially resistant) into the prosthesis area is possible. This risk is even present in cases without a further surgical revision, such as in a non-dry wound or a persistent drain with a subsequent missing skin barrier. A rifampicin resistant Staphylococcus in the joint is a devastating complication, requiring a long-term antibiotic suppression therapy, and oftentimes cannot be eradicated at all. In addition, the 14-day period is used to wait for the intraoperatively gained microbiological culture results. After identifying the definitive culture results, an exact and targeted therapy is possible (1,71).
In two-stage exchanges with a short interval, two weeks of i.v. antibiotics without antibiofilm activity are initiated after the prosthesis explanation, followed by one further week after the reimplantation, and finally ended by 9 weeks of p.o. antibiotics with antibiofilm activity. In contrast, the two-stage exchange with a long interval has 4–6 weeks of p.o. antibiotic without antibiofilm activity between the two-stages. Subsequently, the final p.o. antibiotic application with antibiofilm activity following the i.v. therapy is reduced to 5 weeks. The three-stage exchange consists of seven weeks of continuously i.v. antibiotics administration without antibiofilm activity and 5 weeks of p.o. antibiotics with antibiofilm activity (1,71).
In case of PSII, no specific international guidelines are present concerning route, dose and length of antibiotic administration. Most authors suggest an initial i.v. therapy of 6 to 8 weeks followed by further weeks of oral therapy (9,72,73). Given the limited studies directly analyzing spinal implant infections, several therapy concepts must be transferred from other types of spinal infections like discitis to PSII, in which 3 to 8 weeks of i.v. therapy are proposed (74). Following the initial i.v. antibiotics administration, a switch towards oral antibiotics is an established procedure. In case of spondylodiscitis, oral antibiotics can be given for several weeks and up to three months (75). However, specific schemes like in PJI do not exist. In case of spinal implant preservation, a longer i.v. antibiotics phase might be useful, given the persistence of the infected material, while the complete removal of all infected material might justify a shorter antibiotics administration (76). Some authors suggest that the length of the therapy should primarily be based on the clinical symptoms and lab values such as CRP and ESR (77). However, concerning these parameters one has always to keep in mind their limitations, some of which have been discussed above.
Despite intensive treatment, both, PJI and PSII, bear the risk of persistence, leading to further revisions and a reduced postoperative functionality. This makes infection prevention one of the most important aspects. In PJI, perioperative antimicrobial prophylaxis has been shown to reduce the rate of surgical side infections by up to 80% (78). Similar to that, Barker et al.’s meta-analysis found an effect against gram-positive bacteria in cases of spine surgery (79). Further aspects of prevention include the reduction of risk factors as mentioned above, for example the treatment of an underlying disease or of an immunosuppression. In case of PSII Pull ter Gunne et al. (80) recommended an anterior approach, a decreased blood loss of less than a liter, avoidance of blood transfusions and identification of prior PSII, as prophylactic factors. Ho et al. was able to show that an adequate antibiotic regimen covering the hospital’s specific microbe spectrum had a better outcome following spinal surgery (81). Established prophylaxis strategies in PJI include reduction of skin flora pathogens (82) and antimicrobial-loaded PMMA at prosthesis implantation (83). The usage of vancomycin powder is an established prophylaxis in PSII. In a systematic review and meta-analysis Bakhsheshian et al. were able to show that vancomycin powder reduces the rate of postsurgical spinal infections (84). In contrast, the effectiveness of vancomycin powder for PJI is still controversial with some studies identifying reduced early PJI rates following primary THA and TKA (85), and others increased aseptic wound complications without a decrease in PJI rates following primary knee arthroplasties (86).
Outcome
The comparison of outcome results of PSII and PJI is difficult due to different treatment strategies, different patient characteristics, infection definitions and follow-ups. A general trend shows high reported success rates of PSII treatment using different treatment approaches (Table 3). Compared to these results meta-analyses of PJI indicate worse results with mean re-infection rates of 5% to 15% depending on the involved joint and treatment strategy. A 2016 published meta-analysis from Kunutsor et al. identified a mean re-infection rate of 8.8% (7.2–10.6%) after two-stage revisions in PJI of the knee (108 studies, median follow-up 47 months) (89). An equivalent previous meta-analysis from 2015 by Kunutsor et al. of PJI in the hip reported a re-infection rate of 7.9% (6.2–9.7%) after two-stage exchanges (60 studies, median follow-up 35 months [48–64] (90). Both PJI and PSII show significantly worse results in cases of co-existing or previous tumors involved in the infection (91,92).

Full table
In cases of infection persistence with inoperability or a persistent immunosuppression in the patient, long-term oral antibiotic therapy is the last available treatment. Specific PSII data concerning this type of long-term treatment are lacking (9,63), while the success of this therapy option in PJI is well established (93).
Discussion
When comparing the results of different studies analyzing the risk factors, symptoms, diagnostic and therapeutic approaches for PJI and PSII, several similarities can be noted. Table 4 is showing a brief summary of the detailed comparison of spinal implant and PJI.
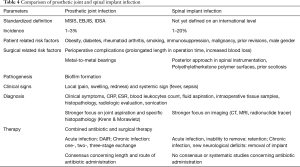
Full table
However, the question remains what kind of implications can be drawn from existing studies dealing with PJI and used for diagnostics and treatment of PSII. In the beginning, it seems obvious that the term “(peri-)spinal implant infection” has not been adequately defined yet when compared to PJI. It is obvious that the term should be discussed and defined on an international basis, similar to the way different organizations and institutions are currently working together to find a consensus definition for PJI (94). In addition to the microbial detection, such a definition should include histopathological criteria and a combination of clinical symptoms and paraclinical parameters. To this point, spine consensus groups are still only using the term “spinal side infection” based on the latest CDC (Center for Disease Control) definition (95).
The comparison of several unspecific risk factors based on general patient associated health aspects like diabetes or immunosuppression did not show significant differences in spine and joint infections. Besides, these factors are known to influence each other or at least being associated with one another, contributing to the difficulty of a single factor analysis. In addition, they not only contribute to the development of an infection, but also to further medical conditions like cardiovascular morbidities. Possible implications deduced from these general and unspecific risk factors are thus limited. In contrast, specific surgical factors like the used material could be used to develop possible solutions for PSII based on existing PJI studies. Current in vitro analyses seem to show that titanium has protective properties against at least some bacteria in cases of both PJI and PSII. This is especially important because the microbes involved in PJI are similar compared to PSII. General in vitro biofilm studies originally intended for research in the field of PJI could therefore also be used to develop new concepts for spinal infections. This is especially of interest given the similar microbe spectrum of PJI and PSII including Staphylococcus aureus and Coagulase-negative Staphylococci. In addition to that, PSII demonstrated a third main spectrum of bacteria with aerobic gram-negative bacilli like E. coli in this study. We state the hypothesis that the additional third main focus of bacteria in spine infections might be associated with the proximity of spinal surgical sites the anus. However, this hypothesis has not been raised in current literature yet. If the proximity of the anus is in fact involved as an additional microbe spectrum in PSII, the entire perioperative antibiotic therapy has to be expanded. In case of a suspected anal focus, gram-negative Enterobacteriaceae (E. coli, Klebsiella, Enterobacter) should additionally be covered via ciprofloxacin (e.g., 2×750 mg, p.o.) (96).
In both PJI and PSII all studies agree that clinical symptoms, patient history and lab values like CRP should always be combined for a final diagnosis. However, while some studies for PJI try to identify a combination of paraclinical signs as being sufficient for a preoperative diagnosis without preoperative microbe detection, none PSII study has yet tried to establish a similar idea (97,98). In general, CRP as screening parameter in both PJI and PSII remains problematic in cases of low-grade infections. Given the different microbe spectrum involved in PJI and PSII, it additionally remains unclear whether differences concerning fast screen lab values between PJI and PSII are caused by the involved prosthesis/implant, the type of infection (low- or high-grade) or the joint itself. Compared to PJI, paraclinical signs like CRP are not analyzed on a larger meta-analysis level specifically for PSII. Concerning preoperative joint aspiration studies should evaluate this procedure in the context of PSII. Compared to PJI imaging, both CT and MRI play a more important role in cases of suspected PSII, considering the limited options concerning preoperative joint aspiration and unspecific clinical signs (9,99). This stronger reliance on imaging in PSII however bears the problem of delayed diagnosis and of uncertainty of the correct diagnosis due to problems when differentiating edema and infection, collections of fluid from uninfected hematoma or seroma, respectively (20).
Overall, the final diagnosis of infection appears to be more difficult in PSII due to limited options compared to PJI, especially when considering the anatomic situation of the spine and thus limited options for tissue sampling. Sonication as a diagnostically tool was initially established in the field of arthroplasty and has now also demonstrated good results in PSII. Therefore, sonication can be considered a classical example of an arthroplasty-based implication for PSII. Similar to that, histopathologic criteria such as the criteria of Krenn & Morawietz (REF) might offer new diagnostical options for PSII as well.
Overall, the treatment of PSII remains to be more problematic and controversial than the one of PJI, due to the much higher risk of instability and permanent neurological damage with every additional revision, difficulties to remove intervertebral cages and disc replacements completely, and different sometimes even contradicting treatment strategies currently in use. In both PJI and PSII an interdisciplinary assessment is essential, given the complexity of cases and the potential complications. In arthroplasty, the differentiation of acute onset and chronic infections is the key towards the right choice of treatment. In chronic cases with likely biofilm formation, a prosthesis or implant exchange for infection consolidation is necessary in most cases, while in acute infections a debridement combined with antibiotics can be the treatment of choice. In PSII, the therapy remains more controversial with more aspects that have to be considered when in the decision-making process, including neuronal damage, stability, acute or chronic infection, and type of implant. Concerning a general antimicrobial and surgical guideline, a standardized treatment protocol and algorithm in use for spinal implant infections is missing. Here, further research with the goal of developing and evaluating such an algorithm seems to be a useful approach.
In general, the outcomes of PSII treatments seem to be better when compared to PJI. We put up the hypothesis that internal fixation in the spine with subsequent reduction in movement and anatomic space (bone fusion) might explain this difference when compared to arthroplasty where a wide range of motion and more anatomic space is necessary. However, due to the lack of meta-analyses and mostly small patient groups, a conclusion cannot be made at this point. In this context, the initially mentioned lack/variability of definitions such as the term “re-infection” or “spinal infection”, different follow-up types, and rates of prior infections and surgeries are a further problem, especially when considering infection numbers following instrumentation.
Conclusions
Diagnosis, prevention and treatment of both PJI and PSII are complex and require an interdisciplinary and specialized setting. Due to their similarities, several concepts from PJI should be transferred to PSII. These include the necessity to define specific terms like re-infection in the context of PSII based on existing definitions of PJI, transfer knowledge from in vitro biofilm studies and studies analyzing different prosthesis surfaces, evaluation of histopathology as an additional standard tool in PSII diagnosis, development of an algorithm for a standardized treatment, and of standardized antibiotic protocols, including long term suppression. Examples in which this kind of knowledge transfer has already been established include the usage of sonication as a diagnostical tool and general aspects about biofilm formation initially evaluated in the context of PJI. In conclusion, results and studies initially developed and evaluated in the context of PJI offer valuable implications for further research and the clinical practice for spinal implant infections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection (PSII)” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-12). The series “Postoperative Spinal Implant Infection (PSII)” was commissioned by the editorial office without any funding or sponsorship. MP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Spine Surgery from Nov 2018 to Nov 2020. CP reports personal fees from Smith & Nephew, personal fees from DePuy/Synthes, personal fees from Zimmer, personal fees from Link, outside the submitted work. TW reports grants from Bonesupport, grants from Heraeus, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014;27:302-45. [Crossref] [PubMed]
- Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;27:61-5.e1. [Crossref] [PubMed]
- Vanhegan IS, Malik AK, Jayakumar P, et al. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Br 2012;94:619-23. [Crossref] [PubMed]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645-54. [Crossref] [PubMed]
- Dale H, Fenstad AM, Hallan G, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 2012;83:449-58. [Crossref] [PubMed]
- Kurtz SM, Ong KL, Lau E, et al. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010;468:52-6. [Crossref] [PubMed]
- Singh JA, Sperling JW, Schleck C, et al. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg 2012;21:1304-9. [Crossref] [PubMed]
- Voloshin I, Schippert DW, Kakar S, et al. Complications of total elbow replacement: a systematic review. J Shoulder Elbow Surg 2011;20:158-68. [Crossref] [PubMed]
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392-403. [Crossref] [PubMed]
- Parvizi J, Zmistowski B, Berbari EF, et al. New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992-4. [Crossref] [PubMed]
- Ochsner P, Borens O, Bodler P, et al. Infections of the musculoskeletal system. Basic principles, prevention, diagnosis and treatment, Swiss Orthopaedics and the Swiss Society for Infectious Diseases Expert Group, 2014. Available online: https://www.heraeus.com/media/media/hme/doc_hme/infection_book/Infections_of_the_musculoskeletal_system_EN.pdf
- Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:e1-25. [Crossref] [PubMed]
- Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res 2013;471:2374-82. [Crossref] [PubMed]
- Nota SP, Braun Y, Ring D, et al. Incidence of surgical site infection after spine surgery: what is the impact of the definition of infection? Clin Orthop Relat Res 2015;473:1612-9. [Crossref] [PubMed]
- Cho OH, Bae IG, Moon SM, et al. Therapeutic outcome of spinal implant infections caused by Staphylococcus aureus: A retrospective observational study. Medicine (Baltimore) 2018;97:e12629. [Crossref] [PubMed]
- Nagashima H, Yamane K, Nishi T, et al. Recent trends in spinal infections: retrospective analysis of patients treated during the past 50 years. Int Orthop 2010;34:395-9. [Crossref] [PubMed]
- Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998;27:1247-54. [Crossref] [PubMed]
- Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum 2008;59:1713-20. [Crossref] [PubMed]
- Jämsen E, Huhtala H, Puolakka T, et al. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am 2009;91:38-47. [Crossref] [PubMed]
- Quaile A.. Infections associated with spinal implants. Int Orthop 2012;36:451-6. [Crossref] [PubMed]
- Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003;98:149-55. [PubMed]
- Soultanis KC, Pyrovolou N, Zahos KA, et al. Late postoperative infection following spinal instrumentation: Stainless steel versus titanium implants. J Surg Orthop Adv 2008;17:193-9. [PubMed]
- Webster TJ, Patel AA, Rahaman MN, et al. Anti-infective and osteointegration properties of silicon nitride, poly (ether ether ketone), and titanium implants. Acta Biomater. 2012;8:4447-54. [Crossref] [PubMed]
- Lenguerrand E, Whitehouse MR, Beswick AD, et al. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis 2018;18:1004-14. [Crossref] [PubMed]
- Bozic KJ, Lau EC, Ong KL, et al. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty 2012;27:37-40. [Crossref] [PubMed]
- Poss R, Thornhill TS, Ewald FC, et al. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res 1984.117-26. [Crossref] [PubMed]
- Inoue D, Kabata T, Kajino Y, et al. Iodine-supported titanium implants have good antimicrobial attachment effects. J Orthop Sci 2019;24:548-51. [Crossref] [PubMed]
- Cahill PJ, Warnick DE, Lee MJ, et al. Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution. Spine (Phila Pa 1976) 2010;35:1211-7. [Crossref] [PubMed]
- Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 2000;13:422-6. [Crossref] [PubMed]
- Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460-5. [Crossref] [PubMed]
- Falavigna A, Righesso O, Traynelis VC, et al. Effect of deep wound infection following lumbar arthrodesis for degenerative disc disease on long-term outcome: A prospective study: Clinical article. J Neurosurg Spine 2011;15:399-403. [Crossref] [PubMed]
- Kowalski TJ, Berbari EF, Huddleston PM, et al. The management and outcome of spinal implant infections: Contemporary retrospective cohort study. Clin Infect Dis 2007;44:913-20. [Crossref] [PubMed]
- Mirovsky Y, Floman Y, Smorgick Y, et al. Management of deep wound infection after posterior lumbar interbody fusion with cages. J Spinal Disord Tech 2007;20:127-31. [Crossref] [PubMed]
- Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am 1985;67:264-73. [Crossref] [PubMed]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167-93. [Crossref] [PubMed]
- Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J 2013;22:2787-99. [Crossref] [PubMed]
- Ting NT, Della Valle CJ. Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J Arthroplasty 2017;32:2047-50. [Crossref] [PubMed]
- Akgün D, Müller M, Perka C, et al. An often-unrecognized entity as cause of recurrent infection after successfully treated two-stage exchange arthroplasty: hematogenous infection. Arch Orthop Trauma Surg 2018;138:1199-206. [Crossref] [PubMed]
- Heggeness MH, Esses SI, Errico T, et al. Late infection of spinal instrumentation by hematogenous seeding. Spine (Phila Pa 1976) 1993;18:492-6. [Crossref] [PubMed]
- Austin MS, Ghanem E, Joshi A, et al. A simple, cost-effective screening protocol to rule out periprosthetic infection. J Arthroplasty 2008;23:65-8. [Crossref] [PubMed]
- Berbari E, Mabry T, Tsaras G, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2010;92:2102-9. [Crossref] [PubMed]
- Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 2010;18:771-2. [Crossref] [PubMed]
- Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445-50. [Crossref] [PubMed]
- Akgün D, Bürger J, Pumberger M, et al. C-reactive protein misdiagnoses delayed postoperative spinal implant infections in patients with low-virulent microorganisms. Eur Spine J 2019;28:2990-5. [Crossref] [PubMed]
- Dobran M, Marini A, Gladi M, et al. Deep spinal infection in instrumented spinal surgery: diagnostic factors and therapy. G Chir 2017;38:124-9. [Crossref] [PubMed]
- Lee YS, Koo KH, Kim HJ, et al. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am 2017;99:2077-84. [Crossref] [PubMed]
- Bedair H, Ting N, Jacovides C, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res 2011;469:34-40. [Crossref] [PubMed]
- Schinsky MF, Della Valle CJ, Sporer SM, et al. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am 2008;90:1869-75. [Crossref] [PubMed]
- Lima AL, Oliveira PR, Carvalho VC, et al. Periprosthetic joint infections. Interdiscip Perspect Infect Dis 2013;2013:542796. [Crossref] [PubMed]
- Ostlere S.. How to image metal-on-metal prostheses and their complications. AJR Am J Roentgenol 2011;197:558-67. [Crossref] [PubMed]
- Tigges S, Stiles RG, Roberson JR. Appearance of septic hip prostheses on plain radiographs. AJR Am J Roentgenol 1994;163:377-80. [Crossref] [PubMed]
- Sofka CM. Current applications of advanced cross-sectional imaging techniques in evaluating the painful arthroplasty. Skeletal Radiology 2007;36:183-93. [Crossref] [PubMed]
- Reinartz P, Mumme T, Hermanns B, et al. Radionuclide imaging of the painful hip arthroplasty: positron-emission tomography versus triple-phase bone scanning. J Bone Joint Surg Br 2005;87:465-70. [Crossref] [PubMed]
- Prinz V, Bayerl S, Renz N, et al. High frequency of low-virulent microorganisms detected by sonication of pedicle screws: a potential cause for implant failure. J Neurosurg Spine 2019;31:424-9. [Crossref] [PubMed]
- Palestro CJ, Love C. Radionuclide imaging of musculoskeletal infection: conventional agents. Semin Musculoskelet Radiol 2007;11:335-52. [Crossref] [PubMed]
- Sertic M, Parkes L, Mattiassi S, et al. The Efficacy of Computed Tomography-Guided Percutaneous Spine Biopsies in Determining a Causative Organism in Cases of Suspected Infection: A Systematic Review. Can Assoc Radiol J 2019;70:96-103. [Crossref] [PubMed]
- Tomas X, Bori G, Garcia S, et al. Accuracy of CT-guided joint aspiration in patients with suspected infection status post-total hip arthroplasty. Skeletal Radiol 2011;40:57-64. [Crossref] [PubMed]
- Isern-Kebschull J, Tomas X, García-Díez AI, et al. Accuracy of Computed Tomography-Guided Joint Aspiration and Computed Tomography Findings for Prediction of Infected Hip Prosthesis. J Arthroplasty 2019;34:1776-82. [Crossref] [PubMed]
- Krenn V, Morawietz L, Perino G, et al. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract 2014;210:779-86. [Crossref] [PubMed]
- Bürger J, Akgün D, Strube P, et al. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J 2019;28:768-74. [Crossref] [PubMed]
- Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine 2010;35:1218-24. [Crossref] [PubMed]
- Rothenberg AC, Wilson AE, Hayes JP, et al. Sonication of Arthroplasty Implants Improves Accuracy of Periprosthetic Joint Infection Cultures. Clin Orthop Relat Res 2017;475:1827-36. [Crossref] [PubMed]
- Quiñones-Hinojosa A, Jun P, Jacobs R, et al. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus 2004;17:E1. [PubMed]
- Karczewski D, Winkler T, Renz N, et al. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Joint J 2019;101-B:132-9. [Crossref] [PubMed]
- Soultanis KC, Pyrovolou N, Zahos K, et al. Late postoperative infection following spinal instrumentation: stainless steel versus titanium implants. J Surg Orthop Adv 2008;17:193-9. [PubMed]
- Kim JI, Suh K, Kim S, et al. Implant removal for the management of infection after instrumented spinal fusion. J Spinal Disord Tech 2010;23:258-65. [Crossref] [PubMed]
- Picada R, Winter R, Lonstein J, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: incidence and management. J Spinal Disord 2000;13:42-5. [Crossref] [PubMed]
- Keller SC, Cosgrove SE, Higgins Y, et al. Role of Suppressive Oral Antibiotics in Orthopedic Hardware Infections for Those Not Undergoing Two-Stage Replacement Surgery. Open Forum Infect Dis 2016;3:ofw176. [Crossref] [PubMed]
- Siqueira MB, Saleh A, Klika AK, et al. Chronic Suppression of Periprosthetic Joint Infections with Oral Antibiotics Increases Infection-Free Survivorship. J Bone Joint Surg Am 2015;97:1220-32. [Crossref] [PubMed]
- Masuda S, Fujibayashi S, Otsuki B, et al. Efficacy of Target Drug Delivery and Dead Space Reduction Using Antibiotic-loaded Bone Cement for the Treatment of Complex Spinal Infection. Clin Spine Surg 2017;30:E1246-50. [Crossref] [PubMed]
- Li C, Renz N, Trampuz A.. Management of Periprosthetic Joint Infection. Hip Pelvis 2018;30:138-46. [Crossref] [PubMed]
- Yin D, Liu B, Chang Y, et al. Management of late-onset deep surgical site infection after instrumented spinal surgery. BMC Surg 2018;18:121. [Crossref] [PubMed]
- Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine 2008;33:289-94. [Crossref] [PubMed]
- Friedman JA, Maher CO, Quast LM, et al. Spontaneous disc space infections in adults. Surg Neurol 2002;57:81-6. [Crossref] [PubMed]
- Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 2008;105:181-7. [PubMed]
- Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine (Phila Pa 1976) 2001;26:1990-6. [Crossref] [PubMed]
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002;34:1342-50. [Crossref] [PubMed]
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008;90:915-9. [Crossref] [PubMed]
- Barker FG. 2nd. Efficacy of prophylactic antibiotic therapy in spinal surgery: A meta-analysis. Neurosurgery 2002;51:391-400. [Crossref] [PubMed]
- Pull ter Gunne AF, Mohamed A, Skolasky R, et al. The presentation, incidence, etiology and treatment of surgical site infections after spinal surgery. Spine 2010;35:1323-8. [Crossref] [PubMed]
- Ho C, Skaggs DL, Weiss JM, et al. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine 2007;32:2739-44. [Crossref] [PubMed]
- Chen AF, Wessel CB, Rao N. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res 2013;471:2383-99. [Crossref] [PubMed]
- Parvizi J, Saleh KJ, Ragland PS, et al. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop 2008;79:335-41. [Crossref] [PubMed]
- Bakhsheshian J, Dahdaleh NS, Lam SK, et al. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg 2015;83:816-23. [Crossref] [PubMed]
- Patel NN, Guild GN 3rd, Kumar AR. Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today 2018;4:479-83. [Crossref] [PubMed]
- Hanada M, Nishikino S, Hotta K, et al. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Arthrosc 2019;27:2322-7. [Crossref] [PubMed]
- Sierra-Hoffman M, Jinadatha C, Carpenter JL, et al. Postoperative instrumented spine infections: a retrospective review. South Med J 2010;103:25-30. [Crossref] [PubMed]
- Glassman SD, Dimar JR, Puno RM, et al. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21:2163-9. [Crossref] [PubMed]
- Kunutsor SK, Whitehouse MR, Lenguerrand E, et al. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0151537. [Crossref] [PubMed]
- Kunutsor SK, Whitehouse MR, Blom AW, et al. Re-Infection Outcomes following One- and Two-Stage Surgical Revision of Infected Hip Prosthesis: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0139166. [Crossref] [PubMed]
- Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 2008;90:62-9. [Crossref] [PubMed]
- Racano A, Pazionis T, Farrokhyar F, et al. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: a systematic review. Clin Orthop Relat Res 2013;471:2017-27. [Crossref] [PubMed]
- Wouthuyzen-Bakker M, Nijman JM, Kampinga GA, et al. Efficacy of Antibiotic Suppressive Therapy in Patients with a Prosthetic Joint Infection. J Bone Jt Infect 2017;2:77-83. [Crossref] [PubMed]
- Signore A, Sconfienza LM, Borens O, et al. Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging 2019;46:971-88. [Crossref] [PubMed]
- Sawyer R, Weistroffer JK, White A. What is the definition of surgical site infection (SSI) in spinal surgery? Spine Consensus. 2.1. DIAGNOSIS: GENERAL PRINCIPLES. Based on: Surgical Site Infection (SSI) Event. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- Pocket Guide to Diagnosis & Treatment of Periprosthetic Joint Infection (PJI). Pro-Implant Foundation. Available online: https://www.pro-implant-foundation.org/
- Karczewski D, Winkler T, Perka C, et al. The Preoperative Microbial Detection is No Prerequisite for the Indication of Septic Revision in Cases of Suspected Periprosthetic Joint Infection. Biomed Res Int 2018;2018:1729605. [Crossref] [PubMed]
- Müller M, Morawietz L, Hasart O, et al. Diagnosis of periprosthetic infection following total hip arthroplasty--evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg Res 2008;3:31. [Crossref] [PubMed]
- Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol 1998;36:2932-9. [Crossref] [PubMed]


