
Full endoscopic unilateral laminotomy for bilateral decompression of the cervical spine: surgical technique and early experience
Introduction
Degenerative cervical spinal stenosis is common in individuals aged 60 years or older with an incidence of approximately 90%, and an expected dramatic rise in prevalence in the near-future (1). Patients with degenerative cervical spinal stenosis are at risk for cervical spondylotic myelopathy (CSM), defined as spinal cord dysfunction due to gradual loss of neurons and myelin from manual compression and spinal cord ischemia (2,3). One of the first symptoms of CSM includes hand dysfunction. As a result, patients can have difficulties feeding and grooming themselves, writing, or performing other fine motor hand tasks. Loss of finger and hand function has the greatest impact on quality of life by far, with five times greater impact than sexual, bladder, bowel, or lower extremity dysfunction (4). The current standard of care for cervical spinal stenosis with severe myelopathy is surgical decompression. Surgical options include anterior cervical discectomy and fusion (ACDF), anterior cervical discectomy and disc arthroplasty, posterior laminoplasty, or posterior cervical decompression and fusion. Options can be limited in the elderly population due to medical comorbidities and high rates of surgical complications. For example, ACDF is a highly effective technique for direct decompression of ventral pathology, however, elderly patients are at high risk to suffer from temporary or permanent postoperative dysphagia (5). Posterior cervical decompression and fusion represents a good option for multilevel stenosis and decompression but requires extensive muscular dissection with the risk of significant blood loss, wound healing issues, non-union and commonly persistent neck pain (6). Finally, laminoplasty is a motion preserving operation with effective decompression but that still requires extensive muscular dissection, and may lead to post-operative kyphosis in older patients (7).
Full-endoscopic spine surgery constitutes an evolution of minimally invasive tubular spine surgery (8). Based on a working channel endoscope and continuous irrigation, full-endoscopic spine surgery was originally developed for the transforaminal approach (9). Advancements in surgical tools, radiofrequency probes and burrs paved the way to adapt the full-endoscopic technique via an interlaminar approach (10,11). While, full-endoscopic spine surgery has been validated as efficacious in the lumbar spine (12-15), there is a paucity of data supporting its role in cervical decompression, with most reports aimed at discectomy and foraminal decompression (16,17).
Here we describe the surgical technique and our early clinical experience using cervical endoscopic unilateral laminotomy for bilateral decompression (CE-ULBD) in a series of elderly patients with severe central stenosis and symptomatic CSM, significant medical comorbidity, and existing cervical deformity.
Methods
After Institutional Review Board approval, we retrospectively queried a prospective spine surgery registry at the University of Washington for CE-ULBD in patients with CSM. From 2014 through 2018, 10 cases of CE-ULBD were identified. Demographic data, operative details, imaging, and patient reported outcomes, including visual analogue scale (VAS) for neck and upper extremity pain, Nurick grade (18), and the modified Japanese Orthopedic Association (mJOA) score (19) were reviewed. The PROCESS guidelines for reporting of case series were used to optimize the manuscript format and content (20).
Surgical technique
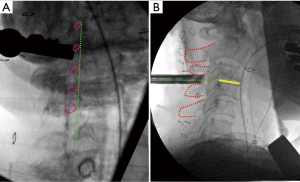
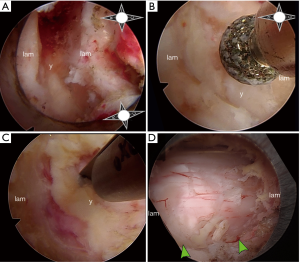
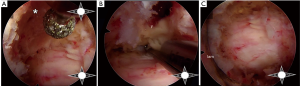
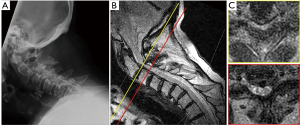
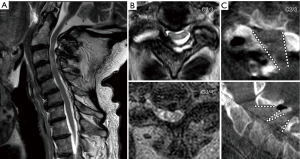
All patients underwent general anesthesia and were positioned prone on a Jackson bed with the head secured in a Mayfield® head holder. All patients had neurophysiological monitoring throughout the case, including motor evoked potentials (MEP) and somatosensory evoked potentials (SSEP). Planning of the mediolateral location of the incision was carried out by marking the medial aspect of the ipsilateral pedicles on an anteroposterior (AP) radiograph (Figure 1A). Utilizing a lateral fluoroscopic image, the rostrocaudal approach trajectory was determined. The trajectory links the posterior aspect of the index level disc space with a line between the index level spinous processes (Figure 1B). A vertical skin incision was marked where the AP and lateral projections intersect. The skin and superficial fascia layers were incised with an #11 blade. Careful sequential dilation of the muscle and facial layers was performed. After palpating the juxtaposed margins of the index level lamina, the tubular retractor was introduced. A working-channel endoscope (iLESSYS® Pro, Joimax® Inc, Irvine, CA), with a 4.7 mm working channel diameter and 7.3mm outer diameter, was utilized with bipolar cautery and the micropunch to remove connective tissue and to visualize the juxtaposed edges of the index level laminae (Figure 2A). The laminotomy was performed along the insertion of the yellow ligament with a diamond burr (Figure 2B). The ligamentum flavum was resected with Kerrison rongeurs and micro-punches (Figure 2C). Identification of the ipsilateral margin of the thecal sac concludes the ipsilateral decompression (Figure 2D). For safe over the top decompression of the contralateral side, the juxtaposed aspects of the index level spinous processes were generously undercut using the diamond burr (Figure 3A). The contralateral lamina and medial facet were undercut with a combination of side-cutting burr and micro-punch. The contralateral buckling yellow ligament was resected using a Kerrison rongeur (Figure 3B). Safe, piecemeal resection of the yellow ligament is contingent on direct visualization of epidural dissection with the heel of the Kerrison. Additionally, a contralateral angle of attack enables maximum decompression with minimal disruption to the ipsilateral facet joint, no disruption of the posterior ligamentous complex (supra- and interspinous ligaments), and safer access to the cervical cord without risking direct instrument injury (21,22). After contralateral decompression, confirmatory AP radiographs were obtained to confirm the contralateral extent of decompression. The fully decompressed spinal cord is shown in Figure 3C.
Statistical analysis
Continuous variables were analyzed as means ± standard error of the mean (SEM). Repeated measurements were compared using a paired-samples T-test. Statistical calculations were carried out using SPSS 26 for Mac.
Results
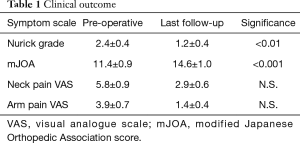
The current study includes 10 patients with symptomatic cervical spinal stenosis who underwent CE-ULBD. The patient cohort consisted of 4 men and 6 women with an average age of 70.2±5.0 years. All patients in our cohort complained of pre-operative neck pain. The VAS for neck pain was on average 5.8±0.9. Loss of hand dexterity was the most common presenting symptom (9/10 patients). Half of the patients in our cohort had severe gait instability which interfered with activities of daily living. More than two thirds of our patient cohort had severe cervical myelopathy as defined as a mJOA score of less than 11 (Table 1). Preoperative imaging revealed one-level spinal stenosis in half of our cohort and 2 level spinal stenosis in the other half. C3/4 was the spinal segment most commonly affected (5/10 patients). Patients underwent CE-ULBD as described. The estimated blood loss was minimal (<10 mL in all cases). Duration of surgery was on average 128±18.4 minutes, and 93.7±11.4 minutes per level. A transient loss of MEP and SSEP was encountered in one patient, with a transient neurological deficit post-operatively that resolved at 1-month follow-up (see case example below). There were no cases of permanent neurological deficit or disability. Average length of stay was 1.2±0.2 days. All patients were discharged back home except for one patient who required assisted living. The average follow-up time in our cohort was 22.0±4.7 months. At that time, the Nurick grades (1.2±0.4, P<0.01) and mJOA scores (14.6±1.0, P<0.001) were significantly improved compared with pre-operative values (Table 1). Patients also experienced a trend towards improvement in their VAS score for neck pain (2.9±0.6), although this did not reach statistical significance.
Full table
Case example #1
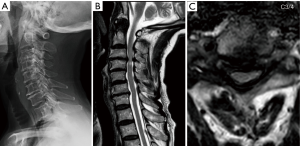
An 84-year-old male presented to clinic with progressive loss of sensation in his hands over the course of 18 months. He was also having difficulty with writing and dexterity of the hands. His past medical history was significant for chronic obstructive pulmonary disease and lung cancer. On exam, he had weak intrinsic hand function bilaterally (3/5) and positive Babinski signs bilaterally (Nurick 4, mJOA 8). His posture showed overt evidence of cervicothoracic kyphosis with a compensatory hyperlordosis of the upper cervical spine to allow for a compensated chin-brow vertical axis. Pre-operative flexion/extension radiographs did not reveal any non-physiological translational movement (Figure 4A). Magnetic resonance imaging (MRI) displayed severe stenosis at C2–3 and moderate stenosis at C3–4 caused by ligamentum flavum buckling (Figure 4B,C). Scoliosis films revealed a sagittal vertical imbalance of 9.3 cm. The patient underwent C2/3 and C3/4 CE-ULBD. Post-operatively his neurologic exam remained stable. Radiographically, his dorsal spinal cord compression was completely relieved with expansion of his cervical cord to physiological shape. Complete facet joint preservation was noted bilaterally as well (Figure 5A,B,C). He was discharged to assisted living after two days. At 12-month follow-up, he had improvement in hand dexterity. There was an improvement of his bilateral hand intrinsic motor strength to 4+/5 and his Nurick and mJOA scores had improved to 3 and 12, respectively.
Case example #2
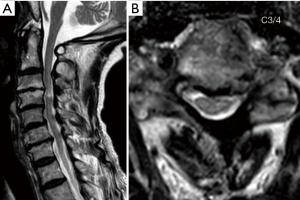
An 80-year-old female presented with severe myelopathy with progressive gait difficulty and upper and lower extremity weakness. She had a past medical history significant for stroke, tachycardia/bradycardia syndrome, and hypertension. On exam she had 3/5 strength in the upper extremities and 4/5 strength in the lower extremities throughout all major muscle groups. Pre-operative radiographs revealed normal alignment with multilevel degenerative disc disease (Figure 6A). Pre-operative MRI demonstrated severe compression at C3/4 with T2 cord signal change immediately below (Figure 6B,C). Her pre-operative Nurick grade was 4 and mJOA was 8. The patient underwent C3/4 CE-ULBD. The blood loss was minimal and operative time was 80 minutes. During contralateral decompression a transient decrease of MEP from the hands and feet bilaterally and SSEP from tibial/median nerves was noted. It was thought to be due to minimal pressure onto the thecal sac during the contralateral decompression. More extensive undercutting of the juxtaposed spinous processes was therefore performed prior to completing the contralateral decompression. Her SSEP and foot MEP recovered, but her hand MEP remained reduced throughout completion of the surgery. Immediately post-operatively, the patient experienced transient worsening of her bilateral hand/arm function. However, her bilateral hand/arm function improved to baseline at 1-month follow-up, and improved beyond her pre-operative function at 3 months follow-up. Post-operative MRI imaging revealed decompression of spinal cord at C3/4 (Figure 7A,B). Two years after her surgery the patient continues to live with her family. While her gait function remained unchanged (Nurick 3), her hand function had somewhat improved resulting in a slightly improved mJOA of 9.
Discussion
In the current report we propose posterior decompression of the cervical spinal cord utilizing full-endoscopic technique in frail, elderly patients.
Changing demographics and rising pathology
With a dramatic increase in the elderly population, the prevalence of degenerative spine disease is steadily rising (23). Half of the patients in our cohort were older than 80 years, and we expect the applications of CE-ULBD to increase with the growth of the elderly population. The patients in the current cohort had a unique, complex pathology, with cervical spinal stenosis largely due to compensatory hyperlordosis in the setting of thoracic kyphosis. Cervical spinal stenosis caused by hyperlordosis, shingling or buckling of the yellow ligament was described by Epstein in 1988 (24). In this report, a traditional laminectomy was recommended, however this procedure is potentially associated with post-laminectomy kyphosis and subsequent neurological deterioration (25). Currently, to avoid this complication, traditional laminectomy is typically combined with arthrodesis (26). Given the underlying spinal deformity in our patient cohort, arthrodesis surgery without correction of spinal deformity would most likely result in subpar functional outcomes (27). Thus, the cervical spine pathology of the current patient cohort would ideally be treated holistically with inclusion of a surgical correction of their cervicothoracic deformity. Traditional posterior three column osteotomies allow for correction of cervical deformity, however, even in a younger patient cohort in the most established medical centers, these surgeries have a complication rate of more than 50% (28). Given the elderly average age of the current patient cohort and their associated co-morbidities, traditional deformity surgery would almost certainly have resulted in a guarded functional outcome for the majority of our patient cohort. The very limited but focused spinal cord decompression allowed for some improvement in the spinal cord dysfunction in most patients, with a short hospitalization and return of these patients to their home. Thus, we propose CE-ULBD as a sensitive treatment alternative in these types of patients.
Full-endoscopic ULBD evolution
Unilateral laminotomy for bilateral access to the spinal canal was first proposed for decompression of the lumbar spine in 1996 by Spetzger and colleagues (29,30). These authors describe the surgical technique of bilateral flavectomy and partial facetectomy via a unilateral laminotomy in both a cadaveric study (29) and in their encouraging initial clinical results (28). Over the top decompression was then combined with tubular retractors (31). This technique allows for preservation of the posterior osseoligamentous complex and results in less destabilization during flexion, extension and axial rotation compared with traditional laminectomies (32). In the lumbar spine, minimally invasive unilateral laminotomy bilateral decompression (ULBD) results in excellent reduction of Oswestry Disability Index, as well as back and leg pain scores, while it is associated with a low complication rate (33,34). With the advance of specialized working channel endoscopes, efficient burrs and endoscopic rongeurs, ULBD was made possible utilizing full-endoscopic technique (35-38). Translating this technique into the cervical spine is challenging due to the presence of the spinal cord, which does not allow similar manipulation compared to the thecal sac of the lumbar spine containing the cauda equina. We chose to utilize a contralateral angle of attack in order to approach the cervical cord as safely as possible (a midline approach risks direct spinal cord injury in the event of over advancing or dropping an instrument). Additionally, this enables preservation of the posterior ligamentous complex and, given a small outer diameter of 7.3 mm, is a muscle sparing approach without any cautery or division of the muscle fibers. Nonetheless, we employed this paramedian approach after many cases of experience, and midline approaches have been reported (39), with the benefit of a more naturally orienting and familiar anatomy for the surgeon. In the current report, electrophysiological monitoring (MEP and SSEP) was carried out in all patients. We report transient loss of signals in one patient, with a transient neurological deficit. Clearly protection of the spinal cord integrity constitutes a major concern for this technique. Several properties of full-endoscopic spinal surgery need to be considered prior to safely embarking on CE-ULBD. First, during the interlaminar approach, three tools have to be safely controlled by the surgeon: the tubular retractor, the endoscope and the tool within the working channel. Loss of control may lead to an impact of any of these tools on the spinal cord with catastrophic consequences. Thus, CE-ULBD should only be carried out by surgeons who are very experienced with full-endoscopic spine surgery. Second, full-endoscopic spine surgery is performed utilizing continuous irrigation. In order to minimize pressure on the dorsal surface of the spinal cord from the constant irrigation, the irrigation pressure should be set at the minimum (typically 40 mmHg), allowing for a good view and clearance of debride. Also, we leave the yellow ligament intact while we perform the ipsilateral hemilaminotomy, and we undercut the adjacent spinous processes in order to shield the dorsal spinal cord surface from the irrigation. Third, the spinous processes need to be undercut generously in order to allow for a working trajectory to the contralateral side without any pressure onto the spinal cord. Following these principles in our cohort, no further events of electrophysiological signal loss were encountered.
In conclusion, CE-ULBD constitutes a novel treatment strategy that will help spine surgeons to offer reasonable treatment strategies for the growing number of octogenarians who maintain an active lifestyle. Full-endoscopic spinal surgery has been associated with a favorable systemic stress response compared with traditional spinal surgery (40). The minimal invasiveness of CE-ULBD allows for a short hospital stay and successful discharge home in the majority of patients. Given the lack of sensitive alternative traditional surgical interventions, the CE-ULBD constitutes an important, novel, minimally invasive intervention for a rapidly growing patient population. Further studies are necessary to define perioperative complications, outcome and mechanical stability following CE-ULBD.
Acknowledgments
The authors appreciate the assistance of Sharon Durfy, PhD, with manuscript preparation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2020.01.03). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Hofstetter reports other from Johnson and Johnson, other from joimax®, other from Globus Medical, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was conducted at the University of Washington Medical Center, Seattle, WA after receiving approval from the University of Washington Institutional Review Board. The IRB waived consent for this retrospective review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cook C, Roman M, Stewart KM, et al. Reliability and diagnostic accuracy of clinical special tests for myelopathy in patients seen for cervical dysfunction. J Orthop Sports Phys Ther 2009;39:172-8. [Crossref] [PubMed]
- Ito T, Oyanagi K, Takahashi H, et al. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine (Phila Pa 1976) 1996;21:827-33. [Crossref] [PubMed]
- Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician 2000;62:1064-70, 1073. [PubMed]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004;21:1371-83. [Crossref] [PubMed]
- Puvanesarajah V, Jain A, Shimer AL, et al. Complications and Mortality Following One to Two-Level Anterior Cervical Fusion for Cervical Spondylosis in Patients Above 80 Years of Age. Spine (Phila Pa 1976) 2017;42:E509-14. [Crossref] [PubMed]
- Asher AL, Devin CJ, Kerezoudis P, et al. Comparison of outcomes following anterior vs posterior fusion surgery for patients with degenerative cervical myelopathy: an analysis from quality outcomes database. Neurosurgery 2019;84:919-26. [Crossref] [PubMed]
- Sakai K, Yoshii T, Hirai T, et al. Cervical Sagittal Imbalance is a Predictor of Kyphotic Deformity After Laminoplasty in Cervical Spondylotic Myelopathy Patients Without Preoperative Kyphotic Alignment. Spine (Phila Pa 1976) 2016;41:299-305. [Crossref] [PubMed]
- Foley KT, Smith MM. Microendoscopic discectomy. Techniques in Neurosurgery 1997;3:301-7.
- Yeung AT. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon's Experience Incorporating Adjunctive Technologies. SAS J 2007;1:108-17. [Crossref] [PubMed]
- Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery 2006;58:ONS59-68; discussion ONS59-68.
- Ruetten S, Komp M, Merk H, et al. Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine 2007;6:521-30. [Crossref] [PubMed]
- Gibson JNA, Subramanian AS, Scott CEH. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur Spine J 2017;26:847-56. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9. [Crossref] [PubMed]
- Chen Z, Zhang L, Dong J, et al. Percutaneous transforaminal endoscopic discectomy compared with microendoscopic discectomy for lumbar disc herniation: 1-year results of an ongoing randomized controlled trial. J Neurosurg Spine 2018;28:300-10. [Crossref] [PubMed]
- McGrath LB, White-Dzuro GA, Hofstetter CP. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression. J Neurosurg Spine 2019;11:1-9. [PubMed]
- Wan Q, Zhang D, Li S, et al. Posterior percutaneous full-endoscopic cervical discectomy under local anesthesia for cervical radiculopathy due to soft-disc herniation: a preliminary clinical study. J Neurosurg Spine 2018;29:351-7. [Crossref] [PubMed]
- Liu C, Liu K, Chu L, et al. Posterior percutaneous endoscopic cervical discectomy through lamina-hole approach for cervical intervertebral disc herniation. Int J Neurosci 2019;129:627-34. [Crossref] [PubMed]
- Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain 1972;95:87-100. [Crossref] [PubMed]
- Benzel EC, Lancon J, Kesterson L, et al. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord 1991;4:286-95. [Crossref] [PubMed]
- Agha RA, Borrelli MR, Farwana R, et al. The PROCESS 2018 statement: Updating Consensus Preferred Reporting Of CasE Series in Surgery (PROCESS) guidelines. Int J Surg 2018;60:279-82. [Crossref] [PubMed]
- Alimi M, Njoku I Jr. Minimally invasive foraminotomy through tubular retractors via a contralateral approach in patients with unilateral radiculopathy. Neurosurgery 2014;10 Suppl 3:436-7. [PubMed]
- Kashlan ON, Kim HS, Khalsa SSS, et al. Percutaneous Endoscopic Contralateral Lumbar Foraminal Decompression via an Interlaminar Approach: 2-Dimensional Operative Video. Oper Neurosurg (Hagerstown) 2020;18:E118-E9. [Crossref] [PubMed]
- Fehlings MG, Tetreault L, Nater A, et al. The Aging of the Global Population: The Changing Epidemiology of Disease and Spinal Disorders. Neurosurgery 2015;77 Suppl 4:S1-5. [Crossref] [PubMed]
- Epstein JA. The surgical management of cervical spinal stenosis, spondylosis, and myeloradiculopathy by means of the posterior approach. Spine (Phila Pa 1976) 1988;13:864-9. [Crossref] [PubMed]
- Albert TJ, Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976) 1998;23:2738-45. [Crossref] [PubMed]
- Kaptain GJ, Simmons NE, Replogle RE, et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg 2000;93:199-204. [PubMed]
- Passias PG, Oh C, Horn SR, et al. Predicting the occurrence of complications following corrective cervical deformity surgery: Analysis of a prospective multicenter database using predictive analytics. J Clin Neurosci 2019;59:155-61. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Lafage R, et al. Three-column osteotomy for correction of cervical and cervicothoracic deformities: alignment changes and early complications in a multicenter prospective series of 23 patients. Eur Spine J 2017;26:2128-37. [Crossref] [PubMed]
- Spetzger U, Bertalanffy H, Naujokat C, et al. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part I: Anatomical and surgical considerations. Acta Neurochir (Wein) 1997;139:392-6. [Crossref] [PubMed]
- Spetzger U, Bertalanffy H, Reinges MH, et al. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part II: Clinical experiences. Acta Neurochir (Wein) 1997;139:397-403. [Crossref] [PubMed]
- Foley KT, Smith MM, Rampersaud YR. Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurg Focus 1999;7:e5. [Crossref] [PubMed]
- Bresnahan L, Ogden AT, Natarajan RN, et al. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976) 2009;34:17-23. [Crossref] [PubMed]
- Alimi M, Hofstetter CP, Pyo SY, et al. Minimally invasive laminectomy for lumbar spinal stenosis in patients with and without preoperative spondylolisthesis: clinical outcome and reoperation rates. J Neurosurg Spine 2015;22:339-52. [Crossref] [PubMed]
- Thomé C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine 2005;3:129-41. [Crossref] [PubMed]
- Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician 2015;18:61-70. [PubMed]
- Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine 2009;10:476-85. [Crossref] [PubMed]
- Wagner R, Telfeian AE, Krzok G, et al. Endoscopic Surgical Technique for Treating Sacral Radiculopathy Secondary to S1 Nerve Compression After Minimally Invasive Sacroiliac Joint Fusion: Technical Note. World Neurosurg 2018;119:349-52. [Crossref] [PubMed]
- Hasan S, Härtl R, Hofstetter CP. The benefit zone of full-endoscopic spine surgery. J Spine Surg 2019;5:S41-56. [Crossref] [PubMed]
- Oshima Y, Takeshita K, Inanami H, et al. Cervical microendoscopic interlaminar decompression through a midline approach in patients with cervical myelopathy: a technical note. J Neurol Surg A Cent Eur Neurosurg 2014;75:474-8. [Crossref] [PubMed]
- Choi KC, Shim HK, Hwang JS, et al. Comparison of Surgical Invasiveness Between Microdiscectomy and 3 Different Endoscopic Discectomy Techniques for Lumbar Disc Herniation. World Neurosurg 2018;116:e750-8. [Crossref] [PubMed]


