Complications of anterior cervical spine surgery: a systematic review of the literature
Introduction
The anterior approach to the cervical spine was first described in the 1950s by Cloward, Robinson, and Smith (1,2), and it has since become a widely utilized technique for central and foraminal decompression. As overall morbidity rates for anterior cervical spine surgery have been historically low, and in some studies lower than those for posterior approaches (3), surgeons have been examining the safety and efficacy of performing it on an outpatient basis (4-6). However, given the complex regional vascular, neural, and aerodigestive anatomy, the anterior approach presents a broad and unique set of potential complications. Furthermore, several of these complications, such as esophageal perforation or vertebral artery pseudoaneurysm, can be fatal if not rapidly recognized.
The current literature of complications associated with the anterior approach to the cervical spine is largely comprised of retrospective studies and case reports. Extant systematic reviews regarding these complications (7-14) comprise individual complications or subsets of patient populations. The most recent review of complications was a focused variant and not systematic (15). In this systematic review, we aim to provide comprehensive qualitative description of the rates, etiologies, management strategies, and outcomes of surgical complications following anterior cervical spine surgery in adults.
Methods
A PubMed search was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for articles containing the terms “anterior cervical” AND (“spine” OR “spinal”) AND “complication.” Studies were limited to those involving adult human subjects, written in the English language, and published over the past 30 years (January 1989 through June 2019). Case reports, retrospective studies, and prospective studies were included. Secondary studies (e.g., existing systematic reviews and meta-analyses), abstracts, letters to the editor, cadaveric studies, studies involving single stage combined anterior and posterior approaches, and studies of non-surgical populations were excluded. Two authors first screened all titles and abstracts and then jointly assessed that the following criteria were consistently applied: the patients underwent anterior-only cervical spine surgery and the patients suffered a surgical complication that was not present preoperatively. The authors then performed a full-text review of the remaining studies. The last search was performed on October 6, 2019.
Data regarding timing, etiology, management, and outcomes of complications were abstracted where available. When reporting pooled incidences in the presence of overlapping patient populations across studies, only the more recent study was included. Presented “n” values reflect the sum of all patients at risk, i.e., the denominator. Comparative analysis was limited given the large number of case reports and heterogeneous patient populations. Qualitative analysis was performed and therefore advanced statistical methodology was not needed for this review.
Results
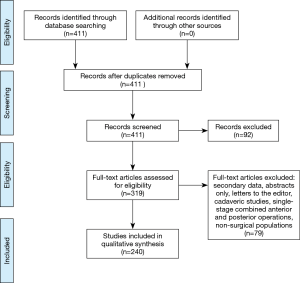
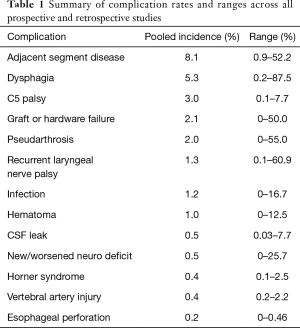
The initial PubMed search yielded 411 records without duplicates. A title and abstract review excluded 92 records, resulting in 319 full-text articles to be assessed for eligibility. After excluding 79 full-text articles, 240 studies remained for inclusion in the systematic review. A flowchart of this process is illustrated in Figure 1. A summary of pooled complication rates and ranges is displayed in Table 1.
Full table
Dysphagia
We identified 1 case report (16), 2 case series (17,18), 10 prospective cohort studies (19-28), and 53 retrospective cohort studies; the retrospective group comprised 2 studies from 1989 to 1999 (29,30), 13 from 2000 to 2009 (31-43), 13 from 2010 to 2014 (44-56), and 25 from 2015 to 2019 (57-81). The case report discussed one patient who presented 8 months after C6–7 ACDF with dysphagia and was found to have a hypopharyngeal diverticulum thought secondary to a spontaneous adhesion of the hypopharynx to the interbody graft. While there was no evidence of perforation, the diverticulum was excised and repaired with fascia lata graft. The dysphagia resolved over 18 months. Across all studies (n=737,041), the rate of postoperative dysphagia at any time point was 5.3%. Among prospective studies (n=1,968), overall rates of postoperative dysphagia ranged from 1.4% to 60.0% with a pooled incidence of 11.5%. Among prospective studies reporting chronic dysphagia (lasting greater than 3 months) (22,23,25,27,28) (n=577), this rate ranged from 0% to 18.0% with an overall rate of chronic dysphagia of 10.1%. Among retrospective studies (n=735,073), overall rates of postoperative dysphagia ranged from 0.21% to 87.5% with a pooled incidence of 5.2%. Among retrospective studies reporting rates of chronic dysphagia (lasting greater than 3 months) (n=2,122) (30-32,34-36,38,41,45,55,57,60-62,74), incidence ranged from 0% to 21.7% with a pooled incidence of 0.8%.
The presence of dysphagia was found to be a predictor for patient re-admission and increased length of stay post-operatively (18). Use of BMP was associated with increased rates of postoperative dysphagia in four retrospective studies (41,42,51,54) (n=332,077) and one prospective study (26) (n=224). The two retrospective studies (52,81) (n=341) that did not find increased rates of dysphagia in the BMP group both reported more severe rates of dysphagia when present in the BMP group compared to the allograft group. De la Garza-Ramos and colleagues (62) in retrospective study of 97 patients found a significantly increased risk of dysphagia in patients undergoing 4- vs. 3-level ACDF (30.8% vs. 12.7%). Another retrospective study of 1,015 patients undergoing ACDF at a single institution (34) found an increased risk of dysphagia in 3-level versus 1- or 2-level ACDF. Lee and colleagues prospectively studied 348 patients undergoing anterior cervical surgery and found that female sex, revision surgery, and greater than two-level operations were associated with greater rates of postoperative dysphagia. A case series of 67 patients with dysphagia after anterior cervical surgery at a single institution (17) found that 70% of the cases were involved more than two levels. Three retrospective studies comparing ACDF to anterior corpectomy (45,57,66) (n=236) found no difference in the rates of postoperative dysphagia. The other two retrospective studies including such a comparison did not identify whether differences were significant (46,47).
A prospective randomized control study (27) found no change in the rate of postoperative dysphagia at 6 weeks, 3 months, or 6 months postoperatively with limiting and monitoring the endotracheal tube cuff pressure to 15 mmHg. The use of local intraoperative steroids was found to reduce the rate of dysphagia at 90 days in patients undergoing ACDF of 3 or more levels, but not in patients undergoing 1- or 2-level ACDF in one retrospective review of the PearlDiver Patient Records Database (80). Local steroids also were shown to reduce Bazaz-Yoo scores at both 6 weeks and 3 months postoperatively in patients undergoing multilevel ACDF in a retrospective case-control study (59). Lower postoperative dysphagia scores in patients who were randomized to preoperative tracheal traction exercises before multilevel ACDF, but this association was not seen for single-level ACDF (28).
Esophageal perforation
We identified 11 retrospective cohort studies (34,56,65,70,71,82-87) that reported rates of esophageal perforation and 31 case reports or case series, this latter group comprising 3 studies from 1989 to 1999 (88-90), 13 from 2000 to 2009 (91-103), and 15 from 2010 to 2019 (104-118). There were no prospective studies. Incidence among the retrospective cohort studies ranged from 0% (71,83,85) to 0.46% (82) with a pooled incidence of 0.2% (n=12,842). Timing of presentation varied widely, ranging from intraoperative discovery (34) to delayed discovery 20 years postoperatively (110). When not discovered intraoperatively, the most common presentation is with dysphagia or odynophagia (82,89,91,93,95,96,98,100,101,103-109,112-114,118), but others include sepsis (109), wound drainage (82,86,102), meningitis (105), quadriparesis secondary to epidural abscess (110), recurrent pneumonia (92,99,103,114), hemoptysis (90), and expectoration of hardware (108).
Management nearly exclusively involves initiation of broad-spectrum antibiotics; exploration; debridement; removal of hardware; primary repair of the esophageal defect; and nutrition via nasogastric tubes, gastrostomy tubes, or jejunostomy tubes (86-88,90-93,96-104,106,107,110-115,117). When primary repair was reinforced, the majority of studies reported use of pedicled muscular flaps (sternocleidomastoid, pectoralis, or strap muscle), though one case series described the successful use of free omental flaps (94) after failure of initial repair. Addition of posterior instrumented fusion (98,112) or placement of an external fixation system (101,115) has been reported for situations in which anterior fusion was not yet achieved. Few exceptions to aggressive surgical management were found. A case report by Shah and colleagues on a patient presenting one week after ACDF described spontaneous esophageal healing after two rounds of debridement but without hardware removal or attempted primary repair (109), another case report of a patient presenting 3.5 years postoperatively after expectorating the anterior construct was not found to have any esophageal defects requiring repair (108), and another case report described successful conservative management with antibiotics and nasogastric tube feeds for 10 days after fistula discovery (95). In a case series of 14 patients with esophageal perforations, Perrone and colleagues reported 2 patients with minimal leaks managed conservatively and who ultimately fully recovered (115).
Culture results are inconsistently reported but are most commonly polymicrobial. The most frequently isolated organisms are Staphylococcus and Streptococcus (91,102,106,107), though Pseudomonas (98,115), Klebsiella (115), Candida (98,106), Acinetobacter (115), and Lactobacillus (105) have also been found. The single case report of meningitis directly associated with esophageal perforation occurred secondary to an esophago-meningeal fistula; Lactobacillus rhamnosus meningitis lead to rapid neurological deterioration and death after a 20-day ICU stay, during which time the surgical intervention was deemed futile (105). Mortality in the literature directly related to the esophageal perforation ranged from 0% (87,114) to 33% (34). Barring death, ultimate resumption of oral intake ranged from 60% (116) to 100% (87,115).
Recurrent laryngeal nerve palsy
We identified 3 case reports (119-121), 6 prospective studies (19,20,24,122-124), and 34 retrospective cohort studies; the retrospective group comprised 7 studies from 1989 to 1999 (29,30,84,125-128), 8 from 2000 to 2009 (18,31,34,37,40,41,129,130), and 19 from 2010 to 2019 (44-49,55-57,62,63,66,73,76,78,85,131-133). Recurrent laryngeal nerve (RLN) palsy was defined as postoperative hoarseness, dysphonia, or vocal cord paralysis. Across retrospective and prospective studies, the pooled incidence of RLN palsy was 1.3%. Among retrospective studies, incidence of RLN palsy ranged from 0.1% to 60.9%, with a pooled incidence of 1.2% (n=26,464). Among prospective studies, incidence of RLN palsy ranged from 0.8% to 5.9%, with a pooled incidence of 2.2% (n=884). Within the studies that reported ultimate outcome of postoperative RLN palsy (n=4,591) (29-31,34,37,41,49,63,84,85,122,126-130), 83.4% of patients experienced partial or complete recovery. The use of a zero-profile cage versus a cage with a separate plating system did not affect the rate of RLN palsy in one prospective study of 104 patients (123) and one retrospective study of 56 patients (31). A retrospective cohort study of 900 patients did find significantly higher rates of RLN palsy in two-level ACDF versus single-level ACDF (129), though two subsequent retrospective studies (n=1,112) (34,62) did not find an association of this complication with the number of operative levels. None of four retrospective studies (n=656) (45-47,66) found a difference in the rates of RLN palsy between multilevel ACDF and corpectomy. The use of recombinant human bone morphogenic protein did not appear to affect rates of RLN palsy in two retrospective studies (41,76). Apfelbaum and colleagues found high rates of RLN palsy in their subset of patients undergoing ACDF for pseudarthrosis (20%) and those undergoing ACDF after any previous anterior neck surgery (10%) (129). Similarly, a subsequent retrospective study of 525 patients who underwent zero profile ACDF did find that RLN palsy was more common in secondary procedures (8%) than primary procedures (2%), a difference that remained significant after multivariate analysis (78).
Infection
We identified 6 case reports (105,134-138), 5 prospective studies (19,20,122-124), and 46 retrospective studies; the retrospective group comprised 4 studies from 1989 to 1999 (29,30,84,125), 4 from 2000 to 2009 (34,35,139,140), 11 from 2010 to 2014 (3,44,46-48,51,53,56,141-143), 10 from 2015 to 2016 (58,62,72,80,133,144-148), and 17 from 2017 to 2019 (66,70,71,78,81,85,131,149-158). Case reports comprised the following: one patient who presented 2 weeks and again 5 months after 3-level ACDF with deep neck space infections growing microaerophilic Streptococci with an odontogenic source (134); one patient who presented 6 weeks after two-level ACDF with osteodiscitis and epidural abscess growing Serratia marcescens (136); one patient who presented 9 months after 2-level corpectomy with a large prevertebral abscess (no culture results) (135); one patient who presented 2 years after 1-level ACDF with a prevertebral abscess growing Streptococcus intermedius (137); one patient who presented 4 years after 1-level ACDF with retropharyngeal abscess growing Streptococcus anginosus, Streptococcus intermedius, Veilonella parcula, and Petptostreptococcus anaerobius (138); and one patient who presented 6 years after 3-level ACDF with meningitis secondary to a esophago-dural fistula growing Lactobaccilius rhamnosus (105). Five of these 6 patients fully recovered after debridement and antibiotic therapy. The case of Lactobacillus meningitis was deemed surgically futile, and the patient died 20 days after presentation despite maximal medical therapy. Across prospective and retrospective studies, the pooled incidence of any infectious complication was 1.2%. Among prospective studies, the incidence of any infectious complication ranged from 0% to 5.0% with a pooled incidence of 1.0% (n=396). Among retrospective studies, the incidence of any infectious complication ranged from 0% to 16.7% with a pooled incidence of 1.2% (n=965,867). Among the studies that specified multiple types of infectious complication (30,44,70,142,143,145,147,154,155,159), bacteremia or sepsis comprised 47.6% of infectious complications. One retrospective analysis of the Medicare database comprising 119,254 patients who underwent anterior cervical fusion found significantly higher rates of postoperative infection in those with rheumatoid arthritis versus those without (157). Preoperative hypoalbuminemia (148), obesity (141), use of BMP (51,81), or use of intraoperative local steroids (80) were not associated with infectious complications. One single-institution retrospective study did not find any difference in infectious complications between 3- and 4-level ACDF (62). Across four retrospective studies, no difference in the rates of infection were found between discectomy and corpectomy cohorts (46,47,57,144).
Adjacent segment disease
We identified 2 prospective studies (20,160) and 12 retrospective studies (35,39,43,47,62,79,132,161-165) that reported rates of adjacent segment disease. Overall, the rate of ASD, inclusive of radiographic cases, symptomatic cases, and those requiring reoperation, was 8.1%. Among prospective studies, rates of ASD ranged from 2.6% to 3.3% with a pooled incidence of 2.9% (n=243). Among retrospective studies, rates of ASD ranged from 0.9% to 52.2% with a pooled incidence of 8.6% (n=2,699). Among studies reporting rates of secondary surgery (20,35,39,43,62,79,132,160,161,163-165), rates of reoperation for ASD ranged from 0% to 12.4% with a pooled incidence of 4.5% (n=2600). Two retrospective studies reported mean times to reoperation of ASD of 32 months (163) and 3.1 years (39). Pre-existing radiographic degeneration of the adjacent level was associated with shortened time to ASD at that level (30 vs. 42 months) in one retrospective study of 1,345 patients (163). Three retrospective studies (43,79,161) found no association between ASD and use of stand-alone cages versus cages with anterior plates (n=404). Rates of ASD were found to be significantly higher in shorter segment fusions in two retrospective studies (163,164) (n=1,447), and non-significant trends toward this association were found in two other retrospective studies (62,162) (n=259).
Pseudarthrosis
We identified 1 case report (166), 1 case series (167), 6 prospective studies (19,20,24,26,160,168), and 31 retrospective studies; the retrospective group comprised 4 studies from 1989 to 1999 (30,125,169,170), 13 from 2000 to 2009 (35,39,41,43,139,161,171-177), and 14 from 2010 to 2019 (52,53,62,74,79,85,123,146,156,159,162,164,165,178). The case report discussed two patients, one 18 months postoperative and one 4 months postoperative from anterior corpectomy, who presented with neck pain after minor trauma and were found to have plate fracture in the setting of known pseudarthrosis. Both patients underwent revision and ultimately achieved complete fusion. The case series included 19 consecutive patients who underwent revision surgery for pseudarthrosis after ACDF. The most common symptom was intractable neck pain with radiculopathy (17 of 19 patients. Mean time to revision for pseudarthrosis was 20 months, and all patients ultimately achieved solid fusion (167). Across all studies, the rate of pseudarthrosis (radiographic, symptomatic, and those requiring revision) at last follow-up was 2.0%. Among prospective studies, the rate of pseudarthrosis ranged from 0.7% to 55.0% with a pooled incidence of 7.0% (n=1,578). Among retrospective studies, the rate of pseudarthrosis ranged from 0% to 23.7% with a pooled incidence of 1.7% (n=32,996). Among studies reporting rates of pseudarthrosis requiring reoperation (20,35,39,41,43,62,74,79,85,139,156,160-162,165,168,171-177), the incidence was 3.0% (n=3,276). Five retrospective studies (79,123,169,170,172,173) (n=379) found no association between use of anterior plating and rates of pseudarthrosis, though one retrospective study of 60 patients did find higher rates of pseudarthrosis in a non-plated ACDF cohort (25%) versus the plated group (0%) (171). Use of BMP was associated with lower rates of pseudarthrosis compared to allograft in two retrospective studies (26,52) (n=860). A retrospective study of 52 patients found no difference in the rate of pseudarthrosis between single level corpectomy and ACDF (173). Two retrospective studies found higher rates of pseudarthrosis with increasing numbers of operated levels (85,164) (n=191), but two other retrospective studies found no such association (62,177) (n=233). One of the retrospective studies that reported higher rates of pseudarthrosis in multilevel ACDF than single-level ACDF noted that no cases warranted reoperation and that that 72.4% of patients with pseudarthrosis at 1 year postoperatively ultimately fused by 2 years (85). A retrospective study of 160 patients who underwent corpectomy found smoking to be an independent risk factor for pseudarthrosis (159) but not in three other retrospective studies (52,170,171) (n=290).
Graft and hardware failure
We identified 11 case reports (179-189), 3 prospective studies (22,24,25), and 42 retrospective cohort studies; the retrospective group comprised 6 studies from 1989 to 1999 (29,30,125,127,128,169),15 from 2000 to 2009 (18,31,32,34,39,43,83,139,140,161,175,176,190-192), and 21 from 2010 to 2019 (3,44-47,56,65,66,74,79,81,149,152,154,156,159,165,193-196). Graft or hardware failure was defined as screw breakage or pullout, screw loosening, plate fracture, graft migration, graft subsidence, or graft fracture. Pseudarthrosis is discussed in a separate section. The case reports discussed graft expectoration 1 day (n=1) (188) and 5 years (n=1) (181) after ACDF, hardware migration into the distal gastrointestinal tract 5 weeks (n=1) (183), 5 months (n=1) (189), and 16 months (n=1) (184) after ACDF, fibular strut allograft fracture 10 months (n=1) and 17 months (n=1) after anterior corpectomy (182), fatigue fracture of the polyurethane sheath 8 years after arthroplasty (n=1) (187), screw loosening and erosion into the pharyngeal mucosa 17 years after ACDF (n=1) (185), retrolisthesis with new myelomalacia at an instrumented level 6 months after corpectomy (n=1) (179), and fracture through an instrumented level 5 years (n=1) (180) and 2 years (n=1) (186) after ACDF. Across prospective and retrospective studies (n=303,714), the overall rate of graft or hardware failure was 2.1%. Among prospective studies (n=565), the rate of graft or hardware failure ranged from 1.2% to 25.0% with a pooled incidence of 3.0%. Among retrospective studies (n=303,149), the rate of graft or hardware failure ranged from 0% to 50.0% with a pooled incidence of 2.1%. Among studies reporting rates of reoperation (22,24,31,34,79,83,127,128,140,169,175,191-194) (n=2,469), the incidence of hardware failure leading to surgical revision ranged from 0% to 100% with a pooled incidence of 22.7%.
Interbody graft fracture was associated with loss of graft height, plate migration, and loss of sagittal alignment in a prospective study of 40 patients undergoing ACDF at a single institution (25). Kaiser and colleagues (161) performed a retrospective study of 233 patients who underwent ACDF with plate stabilization and compared them to 289 historical controls without plating; a significantly higher graft complication rate was seen in the non-plated group. However, in two other retrospective studies (31,169) (n=99), no association was found between graft complication and use of plating. Incidence for graft failure was found to be significantly higher in corpectomy versus multilevel ACDF in two retrospective studies (45,66) (n=190). Two retrospectives studies by Liu and colleagues (46,47) (n=466) did not report measures of statistical significance, but rates of graft or hardware failure were higher in the corpectomy groups than multilevel discectomy groups and the hybrid discectomy-corpectomy groups. In a retrospective study of 120 patients undergoing multilevel ACDF, hybrid discectomy-corpectomy, or corpectomy for multilevel stenosis, the only case of hardware failure occurred in the corpectomy group; however, the difference did not reach statistical significance.
Cerebrospinal fluid leak
We identified 6 case reports (197-202), 2 prospective studies (24,123), and 30 retrospective studies; the retrospective group comprised 1 study from 1989 to 1999 (84), 4 from 2000 to 2009 (24,34,140,203), and 25 from 2010 to 2019 (44-47,49,56,66,70,77-79,123,131-133,146,149,150,159,193,204-208). The case reports discussed the following: 1 patient who suffered quadriparesis immediately postoperative from C5-6 ACDF due to expansion of hydrogel used to repair an intraoperative CSF leak and underwent successful primary repair (197), 4 patients who presented with delayed dysphagia or neck swelling after anterior cervical fusion and underwent successful exploration and repair (198,199,201), one patient who presented 24 hours after C4-5 ACDF with progressive dysphagia and was successfully managed with three lumbar punctures without wound exploration (200), and one patient who experienced wound drainage on postoperative day 1 from C5-7 ACDF and was successfully treated with a cervical epidural blood patch (202). Across prospective and retrospective studies (n=32,229), the rate of CSF leak was 0.5%. Among prospective studies, rates of CSF leak ranged from 0.2% to 1.0% with a pooled incidence of 0.3% (n=592). Among retrospective studies, rates of CSF leak ranged from 0.03% to 7.7% with a pooled incidence of 0.5% (n=31,637). Though the study by Hannallah and colleagues of 1,600 patients undergoing anterior cervical spine surgery found a greater than 3-fold relative risk of CSF leak in corpectomy versus ACDF (203), this association was not found in five moderately sized retrospective studies (45-47,66,193) (n=776). Hannallah and colleagues also found an increased risk of CSF leak in revision versus initial operations (RR 2.75), and in cases of ossification of the posterior longitudinal ligament (RR 13.74). Use of lumbar drains was highly variable, ranging from 0% to 100% (34,140,204).
Hematoma
We identified 5 case reports (209-213), 3 prospective studies (20,122,123), and 37 retrospective studies discussing postoperative cervical wound or epidural hematoma; the retrospective group comprised 2 studies from 1989 to 1999 (30,84), 7 from 2000 to 2009 (18,34,35,39,42,140,214), 11 from 2010 to 2014 (3,44,45,47,51,54,56,159,193,215,216), and 17 from 2015 to 2019 (58,63,66,70,71,73,77,78,146,149,150,156,165,217-220). We separately identified 1 case report (221) and 1 prospective study (222) discussing iliac donor graft site hematoma. The case reports comprised a case of cervical wound hematoma 6 weeks after 1-level ACDF associated with instrumentation settling resulting in neck pain, dysphagia, and shortness of breath (209) (n=1), one case of cervical wound hematoma resulting in neck swelling and dyspnea 16 days after 2-level ACDF secondary to superior thyroid artery hemorrhage requiring hematoma evacuation and endovascular coil embolization (213), and three cases of large ventral epidural hematomas resulting in tetraparesis immediately postoperatively from single level ACDF, single level corpectomy, and 4-level ACDF (210-212) (n=3). All four patients in the case reports underwent evacuation of the hematoma and ultimately recovered. The overall rate of postoperative cervical hematoma across all studies (n=865,340) was 1.0%. Among prospective studies (n=288), the incidence of postoperative cervical hematoma ranged from 0% to 0.7% with a pooled incidence of 0.3%. Among retrospective studies (n=865,052), the incidence of postoperative cervical hematoma ranged from 0% to 12.5% with a pooled incidence of 1.0%. Among studies reporting rates of reoperation for cervical hematoma (18,34,35,165,214,215) (n=1,594), frequency of postoperative hematoma leading to surgical intervention ranged from 0% to 100% with a pooled frequency of 46.1%.
Intentionally kept separate from the above pooled figures of cervical hematomas was the single prospective study of 61 patients undergoing single-level ACDF randomizing patients to non-plated tantalum interbody implant and to autologous iliac bone grafting with plating that reported a 6.1% rate of donor site hematoma requiring evacuation (222). The case report (221) discussed 1 patient who presented 1 month postoperatively from ACDF with a painful left inguinal mass and was found to have a hematoma associated with a pseudoaneurysm of the deep circumflex iliac artery. This was managed with open evacuation of the hematoma and coil embolization of the pseudoaneurysm.
A retrospective study of 37,261 patients in the NSQIP database who underwent ACDF found that a cervical hematoma requiring reoperation significantly increased the risk of re-intubation, ventilator dependence, deep wound infection, and pneumonia (217). This study also identified multilevel ACDF, preoperative INR greater than 1.2, BMI less than 24 kg/m2, and ASA class 3 or greater as independent predictors of postoperative hematoma. Use of BMP was significantly associated with increased risk of hematoma or seroma in a retrospective study of the MarketScan Database and two retrospective studies of the Nationwide Inpatient Sample (42,51,54) (n=332,031). De la Garza Ramos and colleagues reported increased rates of postoperative hematoma in patients with either ESRD or CKD compared to those without kidney disease in a retrospective study of 164,097 patients in the Nationwide Inpatient Sample Database (220). Two retrospective studies (45,66) (n=190) found no difference in the rate of postoperative hematoma between multilevel ACDF and corpectomy for central stenosis. Fountas and colleagues (34) found no association between the number of levels instrumented in ACDF and rate of postoperative hematoma in a retrospective study of 1,105 patients.
Horner syndrome
We identified 1 case report (223), 2 prospective studies (19,20), and 6 retrospective cohort studies (18,30,34,56,84,156) that discussed postoperative Horner syndrome. The case report discussed a patient who was found to have ipsilateral Horner syndrome and contralateral Harlequin syndrome immediately postoperatively from left-sided C6–7 ACDF. An urgent cerebrocervical CT did not demonstrate any hematoma or construct complication, but it did show the surgical drain lying on the ventral surface of the longus colli muscle. Symptoms resolved within hours of drain removal, suggesting local mass effect of the drain on the sympathetic chain was the culprit. Across all studies, the rate of Horner syndrome was 0.4%. Across prospective studies (n=193), the incidence of Horner syndrome ranged from 0.1% to 2.5% with a pooled incidence of 1.0%. Across retrospective studies (n=4,663), the incidence of Horner syndrome ranged from 0.3% to 1.3% with a pooled incidence of 0.3%. The single case of Horner syndrome reported in the retrospective cohort study of 1,105 patients undergoing ACDF at a single institution by Fountas and colleagues (34) resolved completely within 6 weeks.
C5 palsy
We identified 19 retrospective studies (39,40,45-47,57,62,142,144,150,159,196,206,224-229) that discussed postoperative C5 palsy. Across all of the studies (n=5,134) incidence ranged from 0.1% to 7.7% with a pooled incidence of 3.0%. The mean time to recognition of C5 palsy after ACDF or anterior corpectomy in one single institution retrospective study of 176 patients (225) was 1.7 days. Five retrospective studies (45-47,57,144) (n=652) comparing ACDF and anterior corpectomy all found non-significant trends toward higher rates of C5 palsy in the corpectomy groups. One retrospective study of 97 patients undergoing ACDF (62) found no significant difference in rates of postoperative C5 palsy between patients undergoing 4-level or 3-level ACDF. Another retrospective study of 196 patients undergoing ACDF at a single institution by Wagner and colleagues (229) found no significant association between the number of levels operated and the rate of C5 palsy. This study also reported that 6 of the 10 patients with postoperative C5 palsy completely recovered, 2 of the 10 recovered partially, and 2 of the 10 did not recover to any degree by last follow-up (mean 7 months). Eskander and colleagues (225) found that preoperative rotation of the spinal cord was an independent predictor of C5 palsy after ACDF or anterior corpectomy. Those patients who suffered C5 palsy had a mean rotation of 10.3 degrees, and those without this complication had a mean rotation of 2.1 degrees.
Vertebral artery injury
We identified 7 case reports or case series (230-236) and 4 retrospective cohort studies (62,78,140,237) that discussed vertebral artery injury. Across all studies (n=3,884), the incidence of vertebral artery injury ranged from 0.2% to 2.2% with a pooled incidence of 0.4%. One retrospective cohort study of 97 patients undergoing 4- and 3-level ACDF (62) reported a significantly higher risk of vertebral artery injury in 4-level ACDF compared to 3-level ACDF. Among the 16 patients across all studies for whom the clinical history was available, injury was noted intraoperatively in 15 cases (140,230-234,236,237), delayed rupture of a pseudoaneurysm was reported in two cases (234,235), an aberrantly medial vertebral artery was implicated in 3 cases (230,232,233), excessive lateral drilling was implicated in 1 case (234), primary repair without sequelae was achieved in 6 cases (236,237), and arterial ligation without sequelae was achieved in 3 cases (140,236,237). All 3 patients with postoperative neurologic deficit, which were all secondary to PICA territory infarction had undergone endovascular management or intraoperative hemostatic packing (233,235,237). There was 1 intraoperative death due to hypovolemic shock after vertebral artery injury (237).
New or worsened neurologic deficit
We identified 4 case reports (238-241), 1 prospective study (160), and 15 retrospective studies (29,32,34,44,56,72,77,81,83,84,127,133,141,146,206) that discussed new or worsening neurological deficit postoperatively. Across all studies, the overall incidence of new or worsening neurological deficit was 0.5%. The prospective study of 90 patients undergoing ACDF with zero profile cages reported an incidence of 1.1%. Among retrospective studies (n=137,654), the incidence ranged from 0% to 25.7% with a pooled incidence of 0.5%. Among the 10 patients in 6 studies that provided individual clinical history (34,127,238,239,241), 2 patients developed worsened myelopathy due to buckling of the ligamentum flavum and subsequently underwent posterior decompression, 1 patient with OPLL suffered postoperative hemiplegia secondary to spinal cord herniation into a corpectomy defect and subsequently underwent posterior decompression and fusion, 1 patient suffered postoperative hemiplegia secondary to polymethylmethacrylate extrusion into the spinal canal and underwent further anterior decompression with partial recovery, 2 patients suffered worsening myelopathy due to intraoperative cord contusion but recovered with conservative management, 1 patient developed transient Brown Sequard syndrome after dislodging a chip of fibular autograft requiring reoperation for retrieval, 2 patients suffered transient radiculopathy managed conservatively, and 1 patient suffered permanent quadriparesis thought due to intraoperative hypotension. One retrospective study of the California State Inpatient Database (141) found that morbidly obese individuals had a significantly increased odds (OR 3.7) of any postoperative neurological complication compared to those of normal BMI.
Infrequently reported complications
We identified 17 studies for which only case reports existed or fewer than 3 cohort studies existed. Three case reports related to the airway discussed 1 patient with facial cyanosis and hypoxia after multilevel corpectomy requiring reintubation secondary to an overly tight-fitting cervical collar (242), 1 patient with angioedema and tracheal edema in PACU after anterior osteophytectomy requiring tracheostomy (243), and 1 patient with arytenoid dislocation likely secondary to endotracheal intubation for corpectomy resulting in postoperative hoarseness (244). One case series discussed 3 patients with osteoporosis who suffered fractures of the anterior iliac crest at the donor graft site (245). Two case reports related to cervical arthroplasty hardware discussed 1 patient with incidentally discovered, unintended bony fusion across the operated level (246) and another patient with persistent postoperative neck pain likely secondary to excessively augmented segmental mobility (247). Two retrospective studies reported rates of unintended level surgery in ACDF of 0.36% (78) and 2.8% (133). The latter study by Stienen and colleagues did specify that all cases of unintended level surgery were only related to the exposure and that only the intended disc was removed and level fused. Two case reports related to lymphatic injury discussed 1 patient who underwent right-sided ACDF without noted intraoperative thoracic duct injury yet suffered bilateral chylothorax requiring bilateral thoracostomy tube placement (248) and 1 patient who underwent left-sided ACDF and suffered intraoperative thoracic duct injury due to an aberrantly cephalad course of the duct requiring intraoperative ligation with no further sequelae (249). One case report related to cranial nerve injury discussed one patient with permanent hypoglossal nerve palsy thought secondary to intraoperative traction injury during C3 and C4 corpectomy (250). Four case reports related to vascular complications discussed 1 patient with internal jugular vein thrombosis thought secondary to retraction resulting in neck swelling 5 days after single-level ACDF managed successfully with anticoagulation (251), 1 patient with immediate ipsilateral hemothorax of unclear cause after single-level ACDF resulting in dyspnea and tracheal deviation managed with tracheostomy and thoracostomy (252), 1 patient with an unruptured inferior thyroid artery pseudoaneurysm thought secondary to electrocautery resulting in tracheal deviation and neck pain 9 days after single-level ACDF managed successfully with endovascular embolization (253), and 1 patient who suffered a massive brainstem infarct of unclear cause and death after routine single-level ACDF (254).
Discussion
We sought to describe the incidences, etiologies, management strategies, and outcomes of anterior approaches to the cervical spine. Dysphagia is one of the most common postoperative complications, with an incidence of nearly 90% in one identified retrospective study. Fortunately, rates of dysphagia lasting for greater than 3 months appears to be low with a pooled estimate of less than 1%. The most consistent risk factors for postoperative dysphagia were the use of BMP and greater numbers of operated levels. Inconsistent benefit was seen for preoperative tracheal traction exercises and intraoperative local steroid use to decreased rates of dysphagia. Esophageal perforation is an exceedingly rare (pooled incidence of 0.19%) but often life-threatening complication (mortality up to 33%) with delayed presentations including sepsis, aspiration pneumonia, epidural abscess, and meningitis. Cultures are largely polymicrobial, and management is nearly exclusively aggressive with broad-spectrum antibiotics, debridement, hardware removal, esophageal repair, and nutritional bypass. Patients surviving this complication appear to have favorable chances of resuming oral intake, ranging from 60% to 100%.
Recurrent laryngeal nerve palsy manifested by hoarseness or vocal cord hypomobility visualized on laryngoscopy was found to occur at a rate of 1.3% across all studies, and patients experienced rates of partial or complete recovery of greater than 80%. Isolated retrospective studies found higher rates of nerve palsy after revision cases, presumably secondary to the difficulty of navigating disrupted fascial planes and adhesions. Associations with greater operated levels were inconsistent, and no studies found a difference in rates of palsy between multilevel ACDF and corpectomy. Rates of infectious complications were low with an incidence of 0.9% across all studies. While potentially skewed by reporting bias, the finding that nearly half of all infectious complications were bacteremia or sepsis may suggest that the rare infection that occurs postoperatively is often serious and systemic. The only significant association found was in patients with rheumatoid arthritis, a finding that may rationalized by the immunosuppressive effect of several disease modifying anti-rheumatic regimens.
Adjacent segment disease proved to be a heterogeneous entity given inconsistent reporting of radiographic cases, symptomatic cases, or those requiring reoperation. When restricted to only cases requiring reoperation, the pooled incidence was low at 4.5%. The two studies that reported mean times to reoperation provided a similar figure of approximately 3 years. A potentially counterintuitive but largely consistent finding was the lower rate of adjacent segment disease in longer constructs. Though there is creation of a longer lever arm with a longer construct, these also may tend to include the adjacent discs that are at risk of degeneration into the fusion. Further study is necessary to determine the clinical value of prophylactically including radiographically degenerated but asymptomatic adjacent levels, though this strategy has not been proven successful in lumbar spine literature (255).
Rates of pseudarthrosis varied widely given reporting of asymptomatic radiographic graft subsidence to symptomatic neck pain or radiculopathy leading to revision surgery. Nevertheless, the pooled rate remained low at 2.0% at last follow-up. We found significantly lower rates of pseudarthrosis in plated ACDF cohorts, BMP cohorts, and cohorts of fewer operated levels. Smoking was not consistently found to be associated with pseudarthrosis. An encouraging finding in one retrospective study is the high rate of ultimate fusion in patients found to have pseudarthrosis at 1 year postoperatively, though this will need to be verified in prospective studies. Graft and hardware failure proved to be yet another heterogeneous entity with presentations ranging from radiographic screw loosening to graft expectoration or fracture leading to spinal cord injury. The overall incidence was low across all studies at 2.1% with an estimated reoperation rate of 22.7%. Corpectomy compared to ACDF was significantly associated with higher risk of hardware failure in one study with similar non-significant trends in the remainder of the retrospective studies examining the association, suggesting an issue of statistical power.
Cerebrospinal fluid leak was found to be a rare complication with an overall incidence of 0.5%. Significant associations were found with corpectomy versus ACDF, revision versus initial operations, and in patients with ossification of the posterior longitudinal ligament. When discovered in delayed fashion, management was variable, ranging from reoperation to less invasive strategies such as serial lumbar punctures or cervical epidural blood patch. Cervical wound hematoma was found to occur at a rate of 1% with a relatively high frequency leading to reoperation at 46.1%. This is unsurprising given the risks for rapid airway compromise or neurologic deficit in the setting of epidural hematoma. Though retrospective, analyses of large national databases found significant associations of postoperative hematoma with multilevel ACDF, supranormal INR, underweight status, ASA class greater than 3, and impaired kidney function.
As the sympathetic chain lies ventral to the longus colli muscles, they can be damaged at various stages of anterior cervical spine surgery, particularly during exposure of the vertebral bodies (256). The lone case report demonstrated that the sympathetic chain is vulnerable even after dissection is complete; the mass effect of a surgical drain may be sufficient to result in Horner syndrome and Harlequin syndrome. The rates of postoperative Horner syndrome are low in the literature with a pooled rate of 0.4% with some data to suggest recovery can occur even if an immediately reversible cause is not identified. The rate of postoperative C5 palsy across all studies was 3.0%. No association was found with number of operative levels in ACDF, but five retrospective studies reported higher non-significant trends on this complication in corpectomy versus ACDF. Given the low incidence of this complication, this may reflect lack of statistical power. The retrospective study reporting the association of the complication with higher degrees of spinal cord rotation does fit our current paradigm of C5 palsy as a stretch-induced ischemic phenomenon (257).
Vertebral artery injury was reported by few studies and is likely rare with an overall incidence of 0.4%. The case reports and case series illustrated the variable outcomes from no sequelae to PICA territory infarction to intraoperative death. As the vertebral artery is typically within 5 mm of the tip of the uncinate process (258), injury can occur during the decompression with a drill or rongeur as in one of the included case reports. However, aberrant medial location of the vertebral artery can place it at risk even if traditional landmarks are observed, emphasizing the need to scrutinize preoperative imaging.
A multitude of mechanisms have been reported for new or worsened neurologic deficit after anterior cervical surgery, which had a low overall incidence of 0.5%. Severity ranged from transient radiculopathy to permanent quadriparesis, and unsurprisingly, all reported cases with evidence of residual neural compression underwent reoperation. While one study reported higher rates of neurological decline among the morbidly obese compared to normal weight individuals, it remains to be demonstrated whether other approaches to the cervical spine provide a more favorable risk profile in this population if conservative management is not a viable option.
Our review was limited by retrospective data collection and risk of reporting bias, particularly given the preponderance of case reports for several classes of complications.
Conclusions
Morbidity rates in anterior cervical spine surgery are low. Nevertheless, the unique anatomy of the anterior neck presents a wide variety of potential complications—some life-threatening—involving vascular, aerodigestive, neural, and osseous structures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Park is a consultant for and receives royalties from Globus, is a consultant for NuVasive, and receives grants from Depuy and the International Spine Study Group. These relationships pose no conflict of interest with the research reported. Drs. Yee and Swong have no disclosures or conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg 1958;15:602-17. [Crossref] [PubMed]
- Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607-24. [Crossref] [PubMed]
- Memtsoudis SG, Hughes A, Ma Y, et al. Increased in-hospital complications after primary posterior versus primary anterior cervical fusion. Clin Orthop Relat Res 2011;469:649-57. [Crossref] [PubMed]
- Adamson T, Godil SS, Mehrlich M, et al. Anterior cervical discectomy and fusion in the outpatient ambulatory surgery setting compared with the inpatient hospital setting: analysis of 1000 consecutive cases. J Neurosurg Spine 2016;24:878-84. [Crossref] [PubMed]
- Fu MC, Gruskay JA, Samuel AM, et al. Outpatient Anterior Cervical Discectomy and Fusion is Associated With Fewer Short-term Complications in One- and Two-level Cases: A Propensity-adjusted Analysis. Spine (Phila Pa 1976) 2017;42:1044-9. [Crossref] [PubMed]
- Yerneni K, Burke JF, Chunduru P, et al. Safety of Outpatient Anterior Cervical Discectomy and Fusion: A Systematic Review and Meta-Analysis. Neurosurgery 2020;86:30-45. [Crossref] [PubMed]
- Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery: a systematic review. Bone Joint J 2013;95-B:868-73. [Crossref] [PubMed]
- Halani SH, Baum GR, Riley JP, et al. Esophageal perforation after anterior cervical spine surgery: a systematic review of the literature. J Neurosurg Spine 2016;25:285-91. [Crossref] [PubMed]
- Joaquim AF, Murar J, Savage JW, et al. Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J 2014;14:2246-60. [Crossref] [PubMed]
- Noordhoek I, Koning MT, Jacobs WCH, et al. Incidence and clinical relevance of cage subsidence in anterior cervical discectomy and fusion: a systematic review. Acta Neurochir (Wien) 2018;160:873-80. [Crossref] [PubMed]
- Shriver MF, Lewis DJ, Kshettry VR, et al. Pseudoarthrosis rates in anterior cervical discectomy and fusion: a meta-analysis. Spine J 2015;15:2016-27. [Crossref] [PubMed]
- Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia Rates after Anterior Cervical Diskectomy and Fusion: A Systematic Review and Meta-Analysis. Global Spine J 2017;7:95-103. [Crossref] [PubMed]
- Zadegan SA, Jazayeri SB, Abedi A, et al. Corticosteroid Administration to Prevent Complications of Anterior Cervical Spine Fusion: A Systematic Review. Global Spine J 2018;8:286-302. [Crossref] [PubMed]
- Tan TP, Govindarajulu AP, Massicotte EM, et al. Vocal cord palsy after anterior cervical spine surgery: a qualitative systematic review. Spine J 2014;14:1332-42. [Crossref] [PubMed]
- Epstein NE. A Review of Complication Rates for Anterior Cervical Diskectomy and Fusion (ACDF). Surg Neurol Int 2019;10:100. [Crossref] [PubMed]
- Goffart Y, Moreau P, Lenelle J, et al. Traction diverticulum of the hypopharynx following anterior cervical spine surgery. Case report and review. Ann Otol Rhinol Laryngol 1991;100:852-5. [Crossref] [PubMed]
- Leonard R, Belafsky P. Dysphagia following cervical spine surgery with anterior instrumentation: evidence from fluoroscopic swallow studies. Spine (Phila Pa 1976) 2011;36:2217-23. [Crossref] [PubMed]
- Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542-7. [Crossref] [PubMed]
- Ramzi N, Ribeiro-Vaz G, Fomekong E, et al. Long term outcome of anterior cervical discectomy and fusion using coral grafts. Acta Neurochir (Wien) 2008;150:1249-56; discussion 56. [Crossref] [PubMed]
- Skeppholm M, Lindgren L, Henriques T, et al. The Discover artificial disc replacement versus fusion in cervical radiculopathy--a randomized controlled outcome trial with 2-year follow-up. Spine J 2015;15:1284-94. [Crossref] [PubMed]
- Siska PA, Ponnappan RK, Hohl JB, et al. Dysphagia after anterior cervical spine surgery: a prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities. Spine (Phila Pa 1976) 2011;36:1387-91. [Crossref] [PubMed]
- Bose B. Anterior cervical arthrodesis using DOC dynamic stabilization implant for improvement in sagittal angulation and controlled settling. J Neurosurg 2003;98:8-13. [PubMed]
- Lee MJ, Bazaz R, Furey CG, et al. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J 2007;7:141-7. [Crossref] [PubMed]
- Hacker RJ, Cauthen JC, Gilbert TJ, et al. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976) 2000;25:2646-54; discussion 55. [Crossref] [PubMed]
- Falavigna A, Righesso O, Volquind D, et al. Anterior cervical interbody fusion with hydroxyapatite graft: clinical and radiological analysis of graft breakage. Spine (Phila Pa 1976) 2009;34:2769-74. [Crossref] [PubMed]
- Burkus JK, Dryer RF, Arnold PM, et al. Clinical and Radiographic Outcomes in Patients Undergoing Single-level Anterior Cervical Arthrodesis: A Prospective Trial Comparing Allograft to a Reduced Dose of rhBMP-2. Clin Spine Surg 2017;30:E1321-32. [Crossref] [PubMed]
- Kowalczyk I, Ryu WH, Rabin D, et al. Reduced Endotracheal Tube Cuff Pressure to Assess Dysphagia After Anterior Cervical Spine Surgery. J Spinal Disord Tech 2015;28:E552-8. [Crossref] [PubMed]
- Chen Z, Wei X, Li F, et al. Tracheal traction exercise reduces the occurrence of postoperative dysphagia after anterior cervical spine surgery. Spine (Phila Pa 1976) 2012;37:1292-6. [Crossref] [PubMed]
- Caspar W, Barbier DD, Klara PM. Anterior cervical fusion and Caspar plate stabilization for cervical trauma. Neurosurgery 1989;25:491-502. [Crossref] [PubMed]
- Cauthen JC, Kinard RE, Vogler JB, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine (Phila Pa 1976) 1998;23:188-92. [Crossref] [PubMed]
- Hwang SL, Lin CL, Lieu AS, et al. Three-level and four-level anterior cervical discectomies and titanium cage-augmented fusion with and without plate fixation. J Neurosurg Spine 2004;1:160-7. [Crossref] [PubMed]
- Cheng NS, Lau PY, Sun LK, et al. Fusion rate of anterior cervical plating after corpectomy. J Orthop Surg (Hong Kong) 2005;13:223-7. [Crossref] [PubMed]
- Lee MJ, Bazaz R, Furey CG, et al. Influence of anterior cervical plate design on Dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech 2005;18:406-9. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine 2008;9:152-7. [Crossref] [PubMed]
- Ying Z, Wen Y, Xinwei W, et al. Anterior cervical discectomy and fusion for unstable traumatic spondylolisthesis of the axis. Spine (Phila Pa 1976) 2008;33:255-8. [Crossref] [PubMed]
- Morpeth JF, Williams MF. Vocal fold paralysis after anterior cervical diskectomy and fusion. Laryngoscope 2000;110:43-6. [Crossref] [PubMed]
- Stieber JR, Brown K, Donald GD, et al. Anterior cervical decompression and fusion with plate fixation as an outpatient procedure. Spine J 2005;5:503-7. [Crossref] [PubMed]
- Papadopoulos EC, Huang RC, Girardi FP, et al. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine (Phila Pa 1976) 2006;31:897-902. [Crossref] [PubMed]
- Villavicencio AT, Pushchak E, Burneikiene S, et al. The safety of instrumented outpatient anterior cervical discectomy and fusion. Spine J 2007;7:148-53. [Crossref] [PubMed]
- Vaidya R, Carp J, Sethi A, et al. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J 2007;16:1257-65. [Crossref] [PubMed]
- Cahill KS, Chi JH, Day A, et al. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 2009;302:58-66. [Crossref] [PubMed]
- Song KJ, Taghavi CE, Lee KB, et al. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976) 2009;34:2886-92. [Crossref] [PubMed]
- Smith JS, Fu KM, Polly DW Jr, et al. Complication rates of three common spine procedures and rates of thromboembolism following spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2010;35:2140-9. [Crossref] [PubMed]
- Lin Q, Zhou X, Wang X, et al. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J 2012;21:474-81. [Crossref] [PubMed]
- Liu Y, Qi M, Chen H, et al. Comparative analysis of complications of different reconstructive techniques following anterior decompression for multilevel cervical spondylotic myelopathy. Eur Spine J 2012;21:2428-35. [Crossref] [PubMed]
- Liu Y, Hou Y, Yang L, et al. Comparison of 3 reconstructive techniques in the surgical management of multilevel cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2012;37:E1450-8. [Crossref] [PubMed]
- Song KJ, Yoon SJ, Lee KB. Three- and four-level anterior cervical discectomy and fusion with a PEEK cage and plate construct. Eur Spine J 2012;21:2492-7. [Crossref] [PubMed]
- Chen F, He W, Mahaney K, et al. Alternative grafts in anterior cervical fusion. Clin Neurol Neurosurg 2013;115:2049-55. [Crossref] [PubMed]
- Singh K, Marquez-Lara A, Nandyala SV, et al. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2013;38:1820-5. [Crossref] [PubMed]
- Fineberg SJ, Ahmadinia K, Oglesby M, et al. Hospital outcomes and complications of anterior and posterior cervical fusion with bone morphogenetic protein. Spine (Phila Pa 1976) 2013;38:1304-9. [Crossref] [PubMed]
- Lu DC, Tumialan LM, Chou D. Multilevel anterior cervical discectomy and fusion with and without rhBMP-2: a comparison of dysphagia rates and outcomes in 150 patients. J Neurosurg Spine 2013;18:43-9. [Crossref] [PubMed]
- Veeravagu A, Cole T, Jiang B, et al. Revision rates and complication incidence in single- and multilevel anterior cervical discectomy and fusion procedures: an administrative database study. Spine J 2014;14:1125-31. [Crossref] [PubMed]
- Cole T, Veeravagu A, Jiang B, et al. Usage of recombinant human bone morphogenetic protein in cervical spine procedures: analysis of the MarketScan longitudinal database. J Bone Joint Surg Am 2014;96:1409-16. [Crossref] [PubMed]
- Njoku I Jr, Alimi M, Leng LZ, et al. Anterior cervical discectomy and fusion with a zero-profile integrated plate and spacer device: a clinical and radiological study: Clinical article. J Neurosurg Spine 2014;21:529-37. [Crossref] [PubMed]
- Nanda A, Sharma M, Sonig A, et al. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: a single surgeon's experience of 1,576 patients. World Neurosurg 2014;82:1380-7. [Crossref] [PubMed]
- Liu J, Chen X, Liu Z, et al. Anterior cervical discectomy and fusion versus corpectomy and fusion in treating two-level adjacent cervical spondylotic myelopathy: a minimum 5-year follow-up study. Arch Orthop Trauma Surg 2015;135:149-53. [Crossref] [PubMed]
- Cole T, Veeravagu A, Zhang M, et al. Anterior Versus Posterior Approach for Multilevel Degenerative Cervical Disease: A Retrospective Propensity Score-Matched Study of the MarketScan Database. Spine (Phila Pa 1976) 2015;40:1033-8. [Crossref] [PubMed]
- Koreckij TD, Davidson AA, Baker KC, et al. Retropharyngeal Steroids and Dysphagia Following Multilevel Anterior Cervical Surgery. Spine (Phila Pa 1976) 2016;41:E530-4. [Crossref] [PubMed]
- Liu JM, Xiong X, Peng AF, et al. A comparison of local bone graft with PEEK cage versus iliac bone graft used in anterior cervical discectomy and fusion. Clin Neurol Neurosurg 2017;155:30-5. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Seale JA, et al. Clinical Outcomes of Outpatient Cervical Total Disc Replacement Compared With Outpatient Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2017;42:E567-74. [Crossref] [PubMed]
- De la Garza-Ramos R, Xu R, Ramhmdani S, et al. Long-term clinical outcomes following 3- and 4-level anterior cervical discectomy and fusion. J Neurosurg Spine 2016;24:885-91. [Crossref] [PubMed]
- Ba Z, Pan F, Liu X, et al. Do the complications increased in the anterolateral right-side approach to treat the cervical degenerative disorders? A retrospective cohort study. Int J Surg 2017;39:52-6. [Crossref] [PubMed]
- Chen M, Yang S, Yang C, et al. Outcomes observed during a 1-year clinical and radiographic follow-up of patients treated for 1- or 2-level cervical degenerative disease using a biodegradable anterior cervical plate. J Neurosurg Spine 2016;25:205-12. [Crossref] [PubMed]
- Carucci LR, Turner MA, Yeatman CF. Dysphagia secondary to anterior cervical fusion: radiologic evaluation and findings in 74 patients. AJR Am J Roentgenol 2015;204:768-75. [Crossref] [PubMed]
- Li Z, Huang J, Zhang Z, et al. A Comparison of Multilevel Anterior Cervical Discectomy and Corpectomy in Patients With 4-level Cervical Spondylotic Myelopathy: a Minimum 2-year Follow-up Study: Multilevel Anterior Cervical Discectomy. Clin Spine Surg 2017;30:E540-6. [Crossref] [PubMed]
- Liu JM, Tong WL, Chen XY, et al. The incidences and risk factors related to early dysphagia after anterior cervical spine surgery: A prospective study. PLoS One 2017;12:e0173364. [Crossref] [PubMed]
- Olsson EC, Jobson M, Lim MR. Risk factors for persistent dysphagia after anterior cervical spine surgery. Orthopedics 2015;38:e319-23. [Crossref] [PubMed]
- Wu B, Song F, Zhu S. Reasons of Dysphagia After Operation of Anterior Cervical Decompression and Fusion. Clin Spine Surg 2017;30:E554-9. [Crossref] [PubMed]
- Zaki O, Jain N, Yu EM, et al. 30- and 90-Day Unplanned Readmission Rates, Causes, and Risk Factors After Cervical Fusion: A Single-Institution Analysis. Spine (Phila Pa 1976) 2019;44:762-9. [Crossref] [PubMed]
- Yagi K, Nakagawa H, Okazaki T, et al. Noninfectious prevertebral soft-tissue inflammation and hematoma eliciting swelling after anterior cervical discectomy and fusion. J Neurosurg Spine 2017;26:459-65. [Crossref] [PubMed]
- Veeravagu A, Connolly ID, Lamsam L, et al. Surgical outcomes of cervical spondylotic myelopathy: an analysis of a national, administrative, longitudinal database. Neurosurg Focus 2016;40:E11. [Crossref] [PubMed]
- Puvanesarajah V, Jain A, Shimer AL, et al. Complications and Mortality Following One to Two-Level Anterior Cervical Fusion for Cervical Spondylosis in Patients Above 80 Years of Age. Spine (Phila Pa 1976) 2017;42:E509-14. [Crossref] [PubMed]
- Wang B, Lu G, Kuang L. Anterior cervical discectomy and fusion with stand-alone anchored cages versus posterior laminectomy and fusion for four-level cervical spondylotic myelopathy: a retrospective study with 2-year follow-up. BMC Musculoskelet Disord 2018;19:216. [Crossref] [PubMed]
- Tian W, Yu J. The Role of C2-C7 Angle in the Development of Dysphagia After Anterior and Posterior Cervical Spine Surgery. Clin Spine Surg 2017;30:E1306-14. [Crossref] [PubMed]
- Riederman BD, Butler BA, Lawton CD, et al. Recombinant human bone morphogenetic protein-2 versus iliac crest bone graft in anterior cervical discectomy and fusion: Dysphagia and dysphonia rates in the early postoperative period with review of the literature. J Clin Neurosci 2017;44:180-3. [Crossref] [PubMed]
- De la Garza Ramos R, Nakhla J, Nasser R, et al. Volume-Outcome Relationship After 1 and 2 Level Anterior Cervical Discectomy and Fusion. World Neurosurg 2017;105:543-8. [Crossref] [PubMed]
- Staartjes VE, de Wispelaere MP, Schroder ML. Recurrent Laryngeal Nerve Palsy Is More Frequent After Secondary than After Primary Anterior Cervical Discectomy and Fusion: Insights from a Registry of 525 Patients. World Neurosurg 2018;116:e1047-53. [Crossref] [PubMed]
- Tabaraee E, Ahn J, Bohl DD, et al. Comparison of surgical outcomes, narcotics utilization, and costs after an anterior cervical discectomy and fusion: stand-alone cage versus anterior plating. Clin Spine Surg 2017;30:E1201-5. [Crossref] [PubMed]
- Cancienne JM, Werner BC, Loeb AE, et al. The Effect of Local Intraoperative Steroid Administration on the Rate of Postoperative Dysphagia Following ACDF: A Study of 245,754 Patients. Spine (Phila Pa 1976) 2016;41:1084-8. [Crossref] [PubMed]
- Lovasik BP, Holland CM, Howard BM, et al. Anterior cervical discectomy and fusion: comparison of fusion, dysphagia, and complication rates between recombinant human bone morphogenetic protein-2 and beta-tricalcium phosphate. World Neurosurg 2017;97:674-83.e1. [Crossref] [PubMed]
- Zhong ZM, Jiang JM, Qu DB, et al. Esophageal perforation related to anterior cervical spinal surgery. J Clin Neurosci 2013;20:1402-5. [Crossref] [PubMed]
- Huang JJ, Niu CC, Chen LH, et al. Anterior cervical spinal surgery for multilevel cervical myelopathy. Chang Gung Med J 2004;27:531-41. [PubMed]
- Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien) 1989;99:41-50. [Crossref] [PubMed]
- Lee DH, Cho JH, Hwang CJ, et al. What Is the Fate of Pseudarthrosis Detected 1 Year After Anterior Cervical Discectomy and Fusion? Spine (Phila Pa 1976) 2018;43:E23-8. [Crossref] [PubMed]
- Yuan H, Ding H, Hu L, et al. Treatment for early postoperative esophageal fistula complicated with anterior cervical surgery. J Orthop Surg (Hong Kong) 2017;25:2309499016684418. [Crossref] [PubMed]
- Patel NP, Wolcott WP, Johnson JP, et al. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol 2008;69:20-4; discission 4.
- Newhouse KE, Lindsey RW, Clark CR, et al. Esophageal perforation following anterior cervical spine surgery. Spine (Phila Pa 1976) 1989;14:1051-3. [Crossref] [PubMed]
- Kelly MF, Spiegel J, Rizzo KA, et al. Delayed pharyngoesophageal perforation: a complication of anterior spine surgery. Ann Otol Rhinol Laryngol 1991;100:201-5. [Crossref] [PubMed]
- Thomas JP, Finch R. Esophageal erosion. A complication of acrylic fixation in anterior cervical fusion. Spine (Phila Pa 1976) 1991;16:1238-40. [Crossref] [PubMed]
- Gaudinez RF, English GM, Gebhard JS, et al. Esophageal perforations after anterior cervical surgery. J Spinal Disord 2000;13:77-84. [Crossref] [PubMed]
- Witwer BP, Resnick DK. Delayed esophageal injury without instrumentation failure: complication of anterior cervical instrumentation. J Spinal Disord Tech 2003;16:519-23. [Crossref] [PubMed]
- Vrouenraets BC, Been HD, Brouwer-Mladin R, et al. Esophageal perforation associated with cervical spine surgery: report of two cases and review of the literature. Dig Surg 2004;21:246-9. [Crossref] [PubMed]
- Reid RR, Dutra J, Conley DB, et al. Improved repair of cervical esophageal fistula complicating anterior spinal fusion: free omental flap compared with pectoralis major flap. Report of four cases. J Neurosurg 2004;100:66-70. [PubMed]
- Woolley SL, Smith DR. Pharyngeal perforation: a late complication of cervical spine surgery. J Laryngol Otol 2005;119:913-6. [Crossref] [PubMed]
- Konstantakos AK, Temes RT. Delayed esophageal perforation: a complication of anterior cervical spine fixation. Ann Thorac Surg 2005;80:349. [Crossref] [PubMed]
- Summers LE, Gump WC, Tayag EC, et al. Zenker diverticulum: a rare complication after anterior cervical fusion. J Spinal Disord Tech 2007;20:172-5. [Crossref] [PubMed]
- Sahjpaul RL. Esophageal perforation from anterior cervical screw migration. Surg Neurol 2007;68:205-9; discussion 209-10. [Crossref] [PubMed]
- Lu DC, Theodore P, Korn WM, et al. Esophageal erosion 9 years after anterior cervical plate implantation. Surg Neurol 2008;69:310-2; discussion 2-3. [Crossref] [PubMed]
- Benazzo M, Spasiano R, Bertino G, et al. Sternocleidomastoid muscle flap in esophageal perforation repair after cervical spine surgery: concepts, techniques, and personal experience. J Spinal Disord Tech 2008;21:597-605. [Crossref] [PubMed]
- Ardon H, Van Calenbergh F, Van Raemdonck D, et al. Oesophageal perforation after anterior cervical surgery: management in four patients. Acta Neurochir (Wien) 2009;151:297-302; discussion 302. [Crossref] [PubMed]
- Sansur CA, Early S, Reibel J, et al. Pharyngocutaneous fistula after anterior cervical spine surgery. Eur Spine J 2009;18:586-91. [Crossref] [PubMed]
- Dakwar E, Uribe JS, Padhya TA, et al. Management of delayed esophageal perforations after anterior cervical spinal surgery. J Neurosurg Spine 2009;11:320-5. [Crossref] [PubMed]
- Kau RL, Kim N, Hinni ML, et al. Repair of esophageal perforation due to anterior cervical spine instrumentation. Laryngoscope 2010;120:739-42. [Crossref] [PubMed]
- Schmidt M, Maxime V, Pareire F, et al. A lethal case of meningitis due to Lactobacillus rhamnosus as a late complication of anterior cervical spine surgery. J Infect 2011;62:309-10. [Crossref] [PubMed]
- Zairi F, Tetard MC, Thines L, et al. Management of delayed oesophagus perforation and osteomyelitis after cervical spine surgery: review of the literature. Br J Neurosurg 2012;26:185-8. [Crossref] [PubMed]
- Korovessis P, Repantis T, Vitsas V, et al. Cervical spondylodiscitis associated with oesophageal perforation: a rare complication after anterior cervical fusion. Eur J Orthop Surg Traumatol 2013;23 Suppl 2:S159-63. [Crossref] [PubMed]
- Quadri SA, Capua J, Ramakrishnan V, et al. A rare case of pharyngeal perforation and expectoration of an entire anterior cervical fixation construct. J Neurosurg Spine 2017;26:560-6. [Crossref] [PubMed]
- Shah SS, Burks SS, Nguyen DM, et al. Spontaneous healing of a shredded esophagus after ACDF without direct repair. Acta Neurochir (Wien) 2018;160:413-7. [Crossref] [PubMed]
- Park MK, Cho DC, Bang WS, et al. Recurrent esophageal perforation after anterior cervical spine surgery: case report. Eur Spine J 2018;27:515-9. [Crossref] [PubMed]
- Boulahroud O, Choho A, Ajja A. Successfull management of a cervical oesophageal injury after an anterior cervical approach: a case report. Pan Afr Med J 2017;28:274. [Crossref] [PubMed]
- Dobran M, Gladi M, Mancini F, et al. Rare case of anterior cervical discectomy and fusion complication in a patient with Zenker's diverticulum. BMJ Case Rep 2018.11. [PubMed]
- Tian H, Yuan W, Johnson JS, et al. Pharyngoesophageal diverticulum: a delayed complication of anterior cervical spine surgery. Eur Spine J 2011;20 Suppl 2:S211-6. [Crossref] [PubMed]
- Phommachanh V, Patil YJ, McCaffrey TV, et al. Otolaryngologic management of delayed pharyngoesophageal perforation following anterior cervical spine surgery. Laryngoscope 2010;120:930-6. [PubMed]
- Perrone O, Tassi V, Mattioli B, et al. Pharyngo-oesophageal perforation following anterior cervical discectomy and fusion: management and results. Eur J Cardiothorac Surg 2017;51:160-8. [Crossref] [PubMed]
- Simon C, Furstenberg CH, Eichler M, et al. Management of Pharyngeal Fistulas After Anterior Cervical Spine Surgery: A Treatment Algorithm for Severe Complications. Clin Spine Surg 2017;30:E25-30. [Crossref] [PubMed]
- Rueth N, Shaw D, Groth S, et al. Management of cervical esophageal injury after spinal surgery. Ann Thorac Surg 2010;90:1128-33. [Crossref] [PubMed]
- Almre I, Asser A, Laisaar T. Pharyngoesophageal diverticulum perforation 18 years after anterior cervical fixation. Interact Cardiovasc Thorac Surg 2014;18:240-1. [Crossref] [PubMed]
- Muzumdar DP, Deopujari CE, Bhojraj SY. Bilateral vocal cord paralysis after anterior cervical discoidectomy and fusion in a case of whiplash cervical spine injury: a case report. Surg Neurol 2000;53:586-8. [Crossref] [PubMed]
- Manski TJ, Wood MD, Dunsker SB. Bilateral vocal cord paralysis following anterior cervical discectomy and fusion. Case report. J Neurosurg 1998;89:839-43. [Crossref] [PubMed]
- Yerneni K, Burke JF, Nichols N, et al. Delayed Recurrent Laryngeal Nerve Palsy Following Anterior Cervical Discectomy and Fusion. World Neurosurg 2019;122:380-3. [Crossref] [PubMed]
- Chong E, Mobbs RJ, Pelletier MH, et al. Titanium/Polyetheretherketone Cages for Cervical Arthrodesis with Degenerative and Traumatic Pathologies: Early Clinical Outcomes and Fusion Rates. Orthop Surg 2016;8:19-26. [Crossref] [PubMed]
- He S, Feng H, Lan Z, et al. A Randomized Trial Comparing Clinical Outcomes Between Zero-Profile and Traditional Multilevel Anterior Cervical Discectomy and Fusion Surgery for Cervical Myelopathy. Spine (Phila Pa 1976) 2018;43:E259-66. [Crossref] [PubMed]
- Xie Y, Li H, Yuan J, et al. A prospective randomized comparison of PEEK cage containing calcium sulphate or demineralized bone matrix with autograft in anterior cervical interbody fusion. Int Orthop 2015;39:1129-36. [Crossref] [PubMed]
- Shapiro S. Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg 1996;84:161-5. [Crossref] [PubMed]
- Netterville JL, Koriwchak MJ, Winkle M, et al. Vocal fold paralysis following the anterior approach to the cervical spine. Ann Otol Rhinol Laryngol 1996;105:85-91. [Crossref] [PubMed]
- Fessler RG, Steck JC, Giovanini MA. Anterior cervical corpectomy for cervical spondylotic myelopathy. Neurosurgery 1998;43:257-65; discussion 65-7. [Crossref] [PubMed]
- Thalgott JS, Fritts K, Giuffre JM, et al. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine (Phila Pa 1976) 1999;24:1295-9. [Crossref] [PubMed]
- Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976) 2000;25:2906-12. [Crossref] [PubMed]
- Kahraman S, Sirin S, Erdogan E, et al. Is dysphonia permanent or temporary after anterior cervical approach? Eur Spine J 2007;16:2092-5. [Crossref] [PubMed]
- Sun M, Kong L, Jiang Z, et al. Microscope Enhanced the Efficacy and Safety of Anterior Cervical Surgery for Managing Cervical Ossification of the Posterior Longitudinal Ligament. Med Sci Monit 2017;23:3088-94. [Crossref] [PubMed]
- Scholz T, Geiger MF, Mainz V, et al. Anterior Cervical Decompression and Fusion or Posterior Foraminotomy for Cervical Radiculopathy: Results of a Single-Center Series. J Neurol Surg A Cent Eur Neurosurg 2018;79:211-7. [Crossref] [PubMed]
- Stienen MN, Joswig H, Jucker D, et al. Anterior cervical discectomy and fusion: is surgical education safe? Acta Neurochir (Wien) 2015;157:1395-404. [Crossref] [PubMed]
- Pan TJ, Neral M, Gordon Z, et al. Recurrent anterior cervical wound abscesses following cervical corpectomy and fusion surgery from an odontogenic source mimicking an esophageal perforation: a case report. Spine J 2016;16:e399-402. [Crossref] [PubMed]
- Wong DT, Fehlings MG, Massicotte EM. Anterior cervical screw extrusion leading to acute upper airway obstruction: case report. Spine (Phila Pa 1976) 2005;30:E683-6. [Crossref] [PubMed]
- Kulkarni AG, Hee HT. Adjacent level discitis after anterior cervical discectomy and fusion (ACDF): a case report. Eur Spine J 2006;15 Suppl 5:559-63. [Crossref] [PubMed]
- Christiano LD, Goldstein IM. Late prevertebral abscess after anterior cervical fusion. Spine (Phila Pa 1976) 2011;36:E798-802. [Crossref] [PubMed]
- Violon P, Patay Z, Braeckeveldt J, et al. An atypical infectious complication of anterior cervical surgery. Neuroradiology 1997;39:278-81. [Crossref] [PubMed]
- Shapiro S, Connolly P, Donnaldson J, et al. Cadaveric fibula, locking plate, and allogeneic bone matrix for anterior cervical fusions after cervical discectomy for radiculopathy or myelopathy. J Neurosurg 2001;95:43-50. [PubMed]
- Dorai Z, Morgan H, Coimbra C. Titanium cage reconstruction after cervical corpectomy. J Neurosurg 2003;99:3-7. [PubMed]
- Kalanithi PA, Arrigo R, Boakye M. Morbid obesity increases cost and complication rates in spinal arthrodesis. Spine (Phila Pa 1976) 2012;37:982-8. [Crossref] [PubMed]
- Minhas SV, Chow I, Patel AA, et al. Surgeon specialty differences in single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2014;39:1648-55. [Crossref] [PubMed]
- Lovecchio F, Hsu WK, Smith TR, et al. Predictors of thirty-day readmission after anterior cervical fusion. Spine (Phila Pa 1976) 2014;39:127-33. [Crossref] [PubMed]
- Lau D, Chou D, Mummaneni PV. Two-level corpectomy versus three-level discectomy for cervical spondylotic myelopathy: a comparison of perioperative, radiographic, and clinical outcomes. J Neurosurg Spine 2015;23:280-9. [Crossref] [PubMed]
- Bohl DD, Webb ML, Lukasiewicz AM, et al. Timing of Complications After Spinal Fusion Surgery. Spine (Phila Pa 1976) 2015;40:1527-35. [Crossref] [PubMed]
- Harel R, Stylianou P, Knoller N. Cervical Spine Surgery: Approach-Related Complications. World Neurosurg 2016;94:1-5. [Crossref] [PubMed]
- Gruskay JA, Fu M, Basques BA, et al. Factors Affecting Length of Stay and Complications After Elective Anterior Cervical Discectomy and Fusion: A Study of 2164 Patients From The American College of Surgeons National Surgical Quality Improvement Project Database (ACS NSQIP). Clin Spine Surg 2016;29:E34-42. [Crossref] [PubMed]
- Fu MC, Buerba RA, Grauer JN. Preoperative Nutritional Status as an Adjunct Predictor of Major Postoperative Complications Following Anterior Cervical Discectomy and Fusion. Clin Spine Surg 2016;29:167-72. [Crossref] [PubMed]
- Liu H, Ploumis A, Wang S, et al. Treatment of Cervicogenic Headache Concurrent With Cervical Stenosis by Anterior Cervical Decompression and Fusion. Clin Spine Surg 2017;30:E1093-7. [Crossref] [PubMed]
- Hou Y, Liang L, Shi GD, et al. Comparing effects of cervical anterior approach and laminoplasty in surgical management of cervical ossification of posterior longitudinal ligament by a prospective nonrandomized controlled study. Orthop Traumatol Surg Res 2017;103:733-40. [Crossref] [PubMed]
- Purvis TE, Rodriguez HJ, Ahmed AK, et al. Impact of smoking on postoperative complications after anterior cervical discectomy and fusion. J Clin Neurosci 2017;38:106-10. [Crossref] [PubMed]
- Phan K, Kim JS, Lee N, et al. Impact of Insulin Dependence on Perioperative Outcomes Following Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2017;42:456-64. [Crossref] [PubMed]
- Somani S, Di Capua J, Kim JS, et al. Comparing National Inpatient Sample and National Surgical Quality Improvement Program: An Independent Risk Factor Analysis for Risk Stratification in Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2017;42:565-72. [Crossref] [PubMed]
- Rumalla K, Smith KA, Arnold PM. Cervical Total Disc Replacement and Anterior Cervical Discectomy and Fusion: Reoperation Rates, Complications, and Hospital Resource Utilization in 72 688 Patients in the United States. Neurosurgery 2018;82:441-53. [Crossref] [PubMed]
- Segal DN, Wilson JM, Staley C, et al. Outpatient and Inpatient Single-level Cervical Total Disc Replacement: A Comparison of 30-day Outcomes. Spine (Phila Pa 1976) 2019;44:79-83. [Crossref] [PubMed]
- Mullins J, Pojskic M, Boop FA, et al. Retrospective single-surgeon study of 1123 consecutive cases of anterior cervical discectomy and fusion: a comparison of clinical outcome parameters, complication rates, and costs between outpatient and inpatient surgery groups, with a literature review. J Neurosurg Spine 2018;28:630-41. [Crossref] [PubMed]
- Horowitz JA, Puvanesarajah V, Jain A, et al. Rheumatoid Arthritis Is Associated With an Increased Risk of Postoperative Infection and Revision Surgery in Elderly Patients Undergoing Anterior Cervical Fusion. Spine (Phila Pa 1976) 2018;43:E1040-4. [Crossref] [PubMed]
- Khanna R, Kim RB, Lam SK, et al. Comparing Short-term Complications of Inpatient Versus Outpatient Single-level Anterior Cervical Discectomy and Fusion: An Analysis of 6940 Patients Using the ACS-NSQIP Database. Clin Spine Surg 2018;31:43-7. [Crossref] [PubMed]
- Lau D, Chou D, Ziewacz JE, et al. The effects of smoking on perioperative outcomes and pseudarthrosis following anterior cervical corpectomy: Clinical article. J Neurosurg Spine 2014;21:547-58. [Crossref] [PubMed]
- Lonjon N, Favreul E, Huppert J, et al. Clinical and radiological outcomes of a cervical cage with integrated fixation. Medicine (Baltimore) 2019;98:e14097. [Crossref] [PubMed]
- Kaiser MG, Haid RW Jr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery 2002;50:229-36; discussion 236-8. [PubMed]
- Dumont TM, Lin CT, Tranmer BI, et al. Pseudarthrosis failures of anterior subaxial cervical spine fusion using a plate with a single screw per vertebral body: a case series. World Neurosurg 2014;82:225-30. [Crossref] [PubMed]
- Alhashash M, Shousha M, Boehm H. Adjacent segment disease after cervical spine fusion: evaluation of a 70 patient long-term follow-up. Spine (Phila Pa 1976) 2018;43:605-9. [Crossref] [PubMed]
- Komura S, Miyamoto K, Hosoe H, et al. Lower incidence of adjacent segment degeneration after anterior cervical fusion found with those fusing C5-6 and C6-7 than those leaving C5-6 or C6-7 as an adjacent level. J Spinal Disord Tech 2012;25:23-9. [Crossref] [PubMed]
- Narain AS, Hijji FY, Haws BE, et al. Impact of body mass index on surgical outcomes, narcotics consumption, and hospital costs following anterior cervical discectomy and fusion. J Neurosurg Spine 2018;28:160-6. [Crossref] [PubMed]
- Yen CP, Hwang TY, Wang CJ, et al. Fracture of anterior cervical plate implant--report of two cases. Acta Neurochir (Wien) 2005;147:665-7; discussion 667. [Crossref] [PubMed]
- Coric D, Branch CL Jr, Jenkins JD. Revision of anterior cervical pseudoarthrosis with anterior allograft fusion and plating. J Neurosurg 1997;86:969-74. [Crossref] [PubMed]
- Wright IP, Eisenstein SM. Anterior cervical discectomy and fusion without instrumentation. Spine (Phila Pa 1976) 2007;32:772-4; discussion 775. [Crossref] [PubMed]
- Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord 1996;9:202-6. [Crossref] [PubMed]
- Wang JC, McDonough PW, Endow K, et al. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord 1999;12:467-71. [Crossref] [PubMed]
- Wang JC, McDonough PW, Endow KK, et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2000;25:41-5. [Crossref] [PubMed]
- Wang JC, McDonough PW, Kanim LE, et al. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2001;26:643-6; discussion 646-7. [Crossref] [PubMed]
- Wang JC, McDonough PW, Endow KK, et al. A comparison of fusion rates between single-level cervical corpectomy and two-level discectomy and fusion. J Spinal Disord 2001;14:222-5. [Crossref] [PubMed]
- Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976) 2005;30:1756-61. [Crossref] [PubMed]
- Chiang CJ, Kuo YJ, Chiang YF, et al. Anterior cervical fusion using a polyetheretherketone cage containing a bovine xenograftp: three to five-year follow-up. Spine (Phila Pa 1976) 2008;33:2524-428. [Crossref] [PubMed]
- Uribe JS, Sangala JR, Duckworth EA, et al. Comparison between anterior cervical discectomy fusion and cervical corpectomy fusion using titanium cages for reconstruction: analysis of outcome and long-term follow-up. Eur Spine J 2009;18:654-62. [Crossref] [PubMed]
- Jensen WK, Moore TA, Tribus CB, et al. Use of patella allograft for anterior cervical diskectomy and fusion. J Spinal Disord Tech 2009;22:392-8. [Crossref] [PubMed]
- Wang HR, Li XL, Dong J, et al. Skip-level anterior cervical discectomy and fusion with self-locking stand-alone PEEK cages for the treatment of 2 noncontiguous levels of cervical spondylosis. J Spinal Disord Tech 2013;26:E286-92. [Crossref] [PubMed]
- Shen FH, Samartzis D. Careful follow-up after "successful" surgery: postoperative spondylolisthesis after anterior cervical corpectomy and fusion with instrumentation. Surg Neurol 2008;69:637-40; discussion 640. [Crossref] [PubMed]
- Sellin JN, Burks SS, Levi AD. Fracture of fusion mass following anterior cervical plate removal: Case report. J Clin Neurosci 2018;47:128-31. [Crossref] [PubMed]
- Geyer TE, Foy MA. Oral extrusion of a screw after anterior cervical spine plating. Spine (Phila Pa 1976) 2001;26:1814-6. [Crossref] [PubMed]
- Jones J, Yoo J, Hart R. Delayed fracture of fibular strut allograft following multilevel anterior cervical spine corpectomy and fusion. Spine (Phila Pa 1976) 2006;31:E595-9. [Crossref] [PubMed]
- Fujibayashi S, Shikata J, Kamiya N, et al. Missing anterior cervical plate and screws: a case report. Spine (Phila Pa 1976) 2000;25:2258-61. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Machinis T, et al. Extrusion of a screw into the gastrointestinal tract after anterior cervical spine plating. J Spinal Disord Tech 2006;19:199-203. [Crossref] [PubMed]
- Hişmi A, Sahin H, Kinali B, et al. Unexpected Late Complication Causing Dysphagia. Dysphagia 2018;33:481-3. [Crossref] [PubMed]
- Feng R, Loewenstern J, Caridi J. Cervical Burst Fracture in a Patient with Contiguous 2-Level Cervical Stand-Alone Cages. World Neurosurg 2017;105:1041.e1-.e5.
- Fan H, Wu S, Wu Z, et al. Implant failure of Bryan cervical disc due to broken polyurethane sheath: a case report. Spine (Phila Pa 1976) 2012;37:E814-6. [Crossref] [PubMed]
- Cavanagh SP, Tyagi A, Marks P. Extrusion of BOP-B graft orally following anterior cervical discectomy and fusion. Br J Neurosurg 1996;10:417-8. [Crossref] [PubMed]
- Yee GK, Terry AF. Esophageal penetration by an anterior cervical fixation device. A case report. Spine (Phila Pa 1976) 1993;18:522-7. [Crossref] [PubMed]
- Ning X, Wen Y, Xiao-Jian Y, et al. Anterior cervical locking plate-related complications; prevention and treatment recommendations. Int Orthop 2008;32:649-55. [Crossref] [PubMed]
- Lambiris E, Kasimatis GB, Tyllianakis M, et al. Treatment of unstable lower cervical spine injuries by anterior instrumented fusion alone. J Spinal Disord Tech 2008;21:500-7. [Crossref] [PubMed]
- Wang JC, Hart RA, Emery SE, et al. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine (Phila Pa 1976) 2003;28:1016-21; discussion 1021-2. [Crossref] [PubMed]
- Guo Q, Bi X, Ni B, et al. Outcomes of three anterior decompression and fusion techniques in the treatment of three-level cervical spondylosis. Eur Spine J 2011;20:1539-44. [Crossref] [PubMed]
- Landriel FA, Hem S, Goldschmidt E, et al. Polyetheretherketone interbody cages versus autogenous iliac crest bone grafts with anterior fixation for cervical disc disease. J Spinal Disord Tech 2013;26:61-7. [Crossref] [PubMed]
- Yamagata T, Naito K, Arima H, et al. A minimum 2-year comparative study of autologous cancellous bone grafting versus beta-tricalcium phosphate in anterior cervical discectomy and fusion using a rectangular titanium stand-alone cage. Neurosurg Rev 2016;39:475-82. [Crossref] [PubMed]
- Okawa A, Sakai K, Hirai T, et al. Risk factors for early reconstruction failure of multilevel cervical corpectomy with dynamic plate fixation. Spine (Phila Pa 1976) 2011;36:E582-7. [Crossref] [PubMed]
- Thavarajah D, De Lacy P, Hussain R, et al. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine (Phila Pa 1976) 2010;35:E25-6. [Crossref] [PubMed]
- Nair SB, Flood LM, Nath F. An unusual complication of Cloward's procedure presenting to the otolaryngologist. J Laryngol Otol 1998;112:1087-9. [Crossref] [PubMed]
- Schaberg MR, Altman JI, Shapshay SM, et al. Cerebrospinal fluid leak after anterior cervical disc fusion: an unusual cause of dysphagia and neck mass. Laryngoscope 2007;117:1899-901. [Crossref] [PubMed]
- Spennato P, Rapana A, Sannino E, et al. Retropharyngeal cerebrospinal fluid collection as a cause of postoperative dysphagia after anterior cervical discectomy. Surg Neurol 2007;67:499-503. discussion. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Johnston KW. Cerebrospinal fluid fistula secondary to dural tear in anterior cervical discectomy and fusion: case report. Spine (Phila Pa 1976) 2005;30:E277-80. [Crossref] [PubMed]
- Goodman B, Vallabhaneni S, Cubitt B, et al. Transforaminal Epidural Blood Patches for the Treatment of Postsurgical Dural Leaks: Two Case Reports. Pm r 2017;9:83-7. [Crossref] [PubMed]
- Hannallah D, Lee J, Khan M, et al. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am 2008;90:1101-5. [Crossref] [PubMed]
- Elder BD, Theodros D, Sankey EW, et al. Management of Cerebrospinal Fluid Leakage During Anterior Cervical Discectomy and Fusion and Its Effect on Spinal Fusion. World Neurosurg 2016;89:636-40. [Crossref] [PubMed]
- Arshi A, Wang C, Park HY, et al. Ambulatory anterior cervical discectomy and fusion is associated with a higher risk of revision surgery and perioperative complications: an analysis of a large nationwide database. Spine J 2018;18:1180-7. [Crossref] [PubMed]
- Yudoyono F, Cho PG, Park SH, et al. Factors associated with surgical outcomes of cervical ossification of the posterior longitudinal ligament. Medicine (Baltimore) 2018;97:e11342. [Crossref] [PubMed]
- Guerin P, El Fegoun AB, Obeid I, et al. Incidental durotomy during spine surgery: incidence, management and complications. A retrospective review. Injury 2012;43:397-401. [Crossref] [PubMed]
- Syre P, Bohman LE, Baltuch G, et al. Cerebrospinal fluid leaks and their management after anterior cervical discectomy and fusion: a report of 13 cases and a review of the literature. Spine (Phila Pa 1976) 2014;39:E936-43. [Crossref] [PubMed]
- McDowell MM, Parry PV, Agarwal N, et al. Long term delay in onset of prevertebral hematoma following anterior cervical discectomy and fusion: A case report. J Clin Neurosci 2019;62:234-7. [Crossref] [PubMed]
- González-Diaz R, Aunon-Martin I, Ortega-Garcia FJ, et al. Postoperative symptomatic anterior spinal epidural hematoma: complete drainage using corpectomy and a bladder catheter. Spine (Phila Pa 1976) 2016;41:E1368-71. [Crossref] [PubMed]
- Li Y, Zhu QS, Liu JC, et al. Acute cervical epidural hematoma, screw pullout, and esophageal perforation after anterior cervical corpectomy surgery: report of a case. Int Surg 2015;100:334-40. [Crossref] [PubMed]
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of tetraparesis because of postoperative cervical epidural hematoma. Spine J 2010;10:e1-5. [Crossref] [PubMed]
- Yu NH, Jahng TA, Kim CH, et al. Life-threatening late hemorrhage due to superior thyroid artery dissection after anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2010;35:E739-42. [Crossref] [PubMed]
- Vaccaro AR, Robbins MM, Madigan L, et al. Early findings in a pilot study of anterior cervical fusion in which bioabsorbable interbody spacers were used in the treatment of cervical degenerative disease. Neurosurg Focus 2004;16:E7. [Crossref] [PubMed]
- Feng YT, Hwang SL, Lin CL, et al. Safety and resource utilization of anterior cervical discectomy and fusion. Kaohsiung J Med Sci 2012;28:495-9. [Crossref] [PubMed]
- Srinivasan D, La Marca F, Than KD, et al. Perioperative characteristics and complications in obese patients undergoing anterior cervical fusion surgery. J Clin Neurosci 2014;21:1159-62. [Crossref] [PubMed]
- Bovonratwet P, Fu MC, Tyagi V, et al. Incidence, risk factors, and clinical implications of postoperative hematoma requiring reoperation following anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2019;44:543-9. [Crossref] [PubMed]
- Miao W, Ma X, Liang D, et al. Treatment of hematomas after anterior cervical spine surgery: A retrospective study of 15 cases. Neurochirurgie 2018;64:166-70. [Crossref] [PubMed]
- Song KJ, Choi BW, Lee DH, et al. Acute airway obstruction due to postoperative retropharyngeal hematoma after anterior cervical fusion: a retrospective analysis. J Orthop Surg Res 2017;12:19. [Crossref] [PubMed]
- De la Garza Ramos R, Jain A, Nakhla J, et al. Postoperative Morbidity and Mortality After Elective Anterior Cervical Fusion in Patients with Chronic and End-Stage Renal Disease. World Neurosurg 2016;95:480-5. [Crossref] [PubMed]
- Chou AS, Hung CF, Tseng JH, et al. Pseudoaneurysm of the deep circumflex iliac artery: a rare complication at an anterior iliac bone graft donor site treated by coil embolization. Chang Gung Med J 2002;25:480-4. [PubMed]
- Fernández-Fairen M, Sala P, Dufoo M Jr, et al. Anterior cervical fusion with tantalum implant: a prospective randomized controlled study. Spine (Phila Pa 1976) 2008;33:465-72. [Crossref] [PubMed]
- Fringeli Y, Humm AM, Ansorge A, et al. Harlequin sign concomitant with Horner syndrome after anterior cervical discectomy: a case of intrusion into the cervical sympathetic system. J Neurosurg Spine 2017;26:684-7. [Crossref] [PubMed]
- Gandhoke G, Wu JC, Rowland NC, et al. Anterior corpectomy versus posterior laminoplasty: is the risk of postoperative C-5 palsy different? Neurosurg Focus 2011;31:E12. [Crossref] [PubMed]
- Eskander MS, Balsis SM, Balinger C, et al. The association between preoperative spinal cord rotation and postoperative C5 nerve palsy. J Bone Joint Surg Am 2012;94:1605-9. [Crossref] [PubMed]
- Nassr A, Aleem IS, Eck JC, et al. Does Resection of the Posterior Longitudinal Ligament Impact the Incidence of C5 Palsy After Cervical Corpectomy Procedures?: A Review of 459 Consecutive Cases. Spine (Phila Pa 1976) 2017;42:E392-7. [Crossref] [PubMed]
- Odate S, Shikata J, Yamamura S, et al. Extremely wide and asymmetric anterior decompression causes postoperative C5 palsy: an analysis of 32 patients with postoperative C5 palsy after anterior cervical decompression and fusion. Spine (Phila Pa 1976) 2013;38:2184-9. [Crossref] [PubMed]
- Takase H, Murata H, Sato M, et al. Delayed C5 Palsy After Anterior Cervical Decompression Surgery: Preoperative Foraminal Stenosis and Postoperative Spinal Cord Shift Increase the Risk of Palsy. World Neurosurg 2018;120:e1107-19. [Crossref] [PubMed]
- Wagner SC, Sebastian AS, Butler JS, et al. C5 Motor Palsy After Single- and Multi-level Anterior Cervical Diskectomy and Fusion: A Retrospective Review. J Am Acad Orthop Surg 2019;27:e390-4. [Crossref] [PubMed]
- Rosenthal P, Latchaw RE, Kim KD. Anomalous vertebral artery injured during anterior cervical discectomy: a case report. Spine (Phila Pa 1976) 2013;38:E1567-70. [Crossref] [PubMed]
- Wangqin R, Xu K, Mokin M, et al. Covered Stent to Salvage Iatrogenic Vertebral Artery Injury with Uncontrolled Bleeding in the Operating Room Setting. World Neurosurg 2019;122:282-6. [Crossref] [PubMed]
- Khan SA, Coulter I, Marks SM. Iatrogenic vertebral artery injury secondary to vessel tortuosity in a grossly degenerate cervical spine. Br J Neurosurg 2014;28:423-5. [Crossref] [PubMed]
- Eskander MS, Connolly PJ, Eskander JP, et al. Injury of an aberrant vertebral artery during a routine corpectomy: a case report and literature review. Spinal Cord 2009;47:773-5. [Crossref] [PubMed]
- Lo WB, Nagaraja S, Saxena A. Delayed Hemorrhage from an Iatrogenic Vertebral Artery Injury During Anterior Cervical Discectomy and Successful Endovascular Treatment-Report of a Rare Case and Literature Review. World Neurosurg 2017;99:811.e11-8. [Crossref] [PubMed]
- Jecko V, Rue M, Castetbon V, et al. Vertebral artery (V2) pseudo-aneurysm after surgery for cervical schwannoma. How to prevent it and a review of the literature. Neurochirurgie 2015;61:38-42. [Crossref] [PubMed]
- Golfinos JG, Dickman CA, Zabramski JM, et al. Repair of vertebral artery injury during anterior cervical decompression. Spine (Phila Pa 1976) 1994;19:2552-6. [Crossref] [PubMed]
- Burke JP, Gerszten PC, Welch WC. Iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine J 2005;5:508-14; discussion 14. [Crossref] [PubMed]
- Taghvaei M, Meybodi KT, Zeinalizadeh M. Ligamentum Flavum Buckling Causing Immediate Post-Operative Neurological Deterioration After an Anterior Cervical Discectomy: Case Report. Turk Neurosurg 2018;28:678-81. [PubMed]
- He L, Qian Y. Anterior cervical corpectomy and fusion: Spinal cord compression caused by buckled ligamentum flavum. Orthopade 2019;48:170-4. [Crossref] [PubMed]
- Min JH, Jung BJ, Jang JS, et al. Spinal cord herniation after multilevel anterior cervical corpectomy and fusion for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine 2009;10:240-3. [Crossref] [PubMed]
- Jung MY, Shin DA, Hahn IB, et al. Serious complication of cement augmentation for damaged pilot hole. Yonsei Med J 2010;51:466-8. [Crossref] [PubMed]
- Kreisler NS, Durieux ME, Spiekermann BF. Airway obstruction due to a rigid cervical collar. J Neurosurg Anesthesiol 2000;12:118-9. [Crossref] [PubMed]
- Krnacik MJ, Heggeness MH. Severe angioedema causing airway obstruction after anterior cervical surgery. Spine (Phila Pa 1976) 1997;22:2188-90. [Crossref] [PubMed]
- Zhong Z, Hu J, Wu N, et al. Prolonged Hoarseness Caused by Arytenoid Dislocation After Anterior Cervical Corpectomy and Fusion. Spine (Phila Pa 1976) 2016;41:E174-7. [Crossref] [PubMed]
- Al-Sayyad MJ, Abdulmajeed TM. Fracture of the anterior iliac crest following autogenous bone grafting. Saudi Med J 2006;27:254-8. [PubMed]
- Parkinson JF, Sekhon LH. Cervical arthroplasty complicated by delayed spontaneous fusion. Case report. J Neurosurg Spine 2005;2:377-80. [Crossref] [PubMed]
- Gautschi OP, Corniola MV, Stienen MN, et al. Postoperative segmental hypermobility after cervical arthroplasty: A possible pathomechanism for outcome failure. J Clin Neurosci 2015;22:1194-6. [Crossref] [PubMed]
- Bae JS, Park JH, Jang IT. Bilateral chylothorax following anterior cervical spine surgery. Acta Neurochir (Wien) 2017;159:2019-21. [Crossref] [PubMed]
- Hart AK, Greinwald JH Jr, Shaffrey CI, et al. Thoracic duct injury during anterior cervical discectomy: a rare complication. Case report. J Neurosurg 1998;88:151-4. [Crossref] [PubMed]
- Sengupta DK, Grevitt MP, Mehdian SM. Hypoglossal nerve injury as a complication of anterior surgery to the upper cervical spine. Eur Spine J 1999;8:78-80. [Crossref] [PubMed]
- Karim A, Knapp J, Nanda A. Internal jugular venous thrombosis as a complication after an elective anterior cervical discectomy: case report. Neurosurgery 2006;59:E705; discussion E705.
- Harhangi BS, Menovsky T, Wurzer HA. Hemothorax as a complication after anterior cervical discectomy: case report. Neurosurgery 2005;56:E871. discussion E. [Crossref] [PubMed]
- Jenis LG, Leclair WJ. Late vascular complication with anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 1994;19:1291-3. [Crossref] [PubMed]
- Tsai YF, Doufas AG, Huang CS, et al. Postoperative coma in a patient with complete basilar syndrome after anterior cervical discectomy. Can J Anaesth 2006;53:202-7. [Crossref] [PubMed]
- Throckmorton TW, Hilibrand AS, Mencio GA, et al. The impact of adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine (Phila Pa 1976) 2003;28:2546-50. [Crossref] [PubMed]
- Yin Z, Yin J, Cai J, et al. Neuroanatomy and clinical analysis of the cervical sympathetic trunk and longus colli. J Biomed Res 2015;29:501-7. [PubMed]
- Andelman SM, McAnany SJ, Qureshi SA, et al. Bilateral C5 Motor Palsy after Anterior Cervical Decompression and Fusion: A Case Report and Review of the Literature. Int J Spine Surg 2017;11:14. [Crossref] [PubMed]
- Kim SH, Lee JH, Kim JH, et al. Anatomical morphometric study of the cervical uncinate process and surrounding structures. J Korean Neurosurg Soc 2012;52:300-5. [Crossref] [PubMed]