
Evolution of endoscopic transforaminal lumbar approach for degenerative lumbar disease
Introduction
The increase in life expectancy and the change in lifestyle caused an increase in incidence of degenerative spinal disease along with the increase number of surgeries performed in degenerative spine conditions. There is a rise in minimally invasive spine surgery along with these increases in trend. Endoscopic spine surgery is the most advanced form of minimally invasive spine surgery, and has recently undergone rapid development (1,2). For endoscopic spine surgery with aim of minimal soft tissue damage during the surgery, the transforaminal approach has been limited to indications due to itsoptimized approach and obstacles of bony or neural structures. Since the initial transforaminal approach is based on the ‘inside out’ technique, there were many limitations on the indications. ‘Outside-in’ technique has been developed to address the limitation of ‘inside-out’ technique. However, the ‘outside-in’ approach was not free from anatomical obstacles and the ‘mobile outside-in’ approach was developed to further resolve the limitations.
Current review article provides an overview of the evolution of transforaminal approach with its future application in the endoscopic spine surgery.
History
Endoscopic spinal surgery began as percutaneous discectomy as blind image guided procedure around 1960. For ease of understanding we have divided the time period depending on the timing from when visualized endoscopic spine surgery was discovered.
Pre visualization era
First reported attempt of percutaneous discectomy done by Smith (3-5) in 1963 by injecting chymopapain an enzyme derived from papaya plant into the disc space. He called it as a chemonucleolysis. In 1975, Hijikata (6) from Japan did percutaneous manual discectomy with some success while in 1984 Ascher first used laser to do percutaneous discectomy. Onik (7) proposed automated percutaneous lumbar discectomy in 1985. Kambin and colleagues (8,9) developed a technique to remove nucleus pulposus with the help of 5 mm Craig’s cannula in the 70’s. Thus percutaneous discectomy started as blind fluoroscopic guided technique based on principles of indirect decompression; as pathology was considered only due to pressure exerted by herniated disc on exiting or traversing nerve root. However this techniques gain popularity due to least invasive with more effective and faster results compared to conservative management. In 1990, Kambin (10,11) introduced the concept of “safe zone” over dorso lateral aspect of disc for transforaminal approach which was a turning point in the history of endoscopic spine surgery. Mirkovic et al. (12) found in their study over 12 cadaveric specimen the estimated dimensions of safe zone in L1–S1 foramen, according to study 6.3 mm working cannula is safe in mid pedicular line and 7.5 mm working cannula is safe for medial pedicular line.
Post visualization era
In 1986, Kambin and Sampson (9) developed full endoscopic (FE) technique using arthroscope. It was the first attempt to visualise the disc pathology through endoscope. At parallel time, Destandau (13) developed endoscope assisted spine surgery with his custom made endoscope aided system in 1999.
The original ‘inside-out’ technique described by Yeung (14) with development of Yeung endoscopic spine surgery system (YESS) in 2001. It was based on principle of in vivo visualization of pain generator inside the foramen by provocative method and replaced already existing indirect percutaneous discectomy techniques. ‘Outside-in’ technique was first introduced and popularized by Hoagland et al. (15-18) as Thessys technique. It allowed removal of a wider range of lumbar disc herniation (LDH). In 2005, Lee et al. (19) in 2007 introduced ‘half and half’ view of epiduroscopic approach to minimize the risk of injury to neural structures. The bony limitations while targeting inferior and superior migrated fragments was overcame by the introduction of endoscopic drill and foraminoplasty approach by Choi et al. (20). In 2008, Ruetten et al. (21) described the interlaminar access with the same instruments, mainly forL5-S1 access in 2007. In 2017, Kim et al. introduced the ‘mobile outside-in’ technique for migrated disc herniation (22) (Table 1).
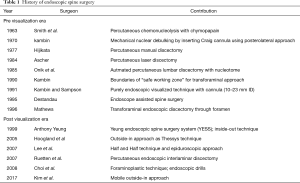
Full table
Anatomical structures of transforaminal area
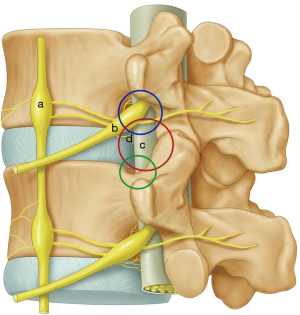
Kambin (10,11) from Philadelphia in his extensive cadaveric study defined “safe working zone” for transforaminal approach between traversing and exiting nerve root over dorso-lateral surface. He also described position of spinal needle in safe zone on antero-posterior and lateral radiographic view. Kambin’s triangle is a three-dimensional anatomical right angle triangle located over the dorso-lateral intervertebral disc of the lumbar spine. Kambin’s triangle’s base formed by superior end plate of inferior vertebra, medial border by traversing nerve root and dura covered with facet joint and hypotenuse formed by exiting nerve root with DRG (9). It contents loose adipose tissue with few epidural veins (23,24).
Anatomical considerations for migrated disc
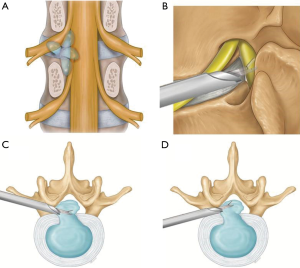
Transforaminal approach provides adequate access for removal of non-migrated or low grade migrated disc herniation but not for high grade disc herniation (25-29). Biggest difficulty is encountered in obtaining optimal trajectory which is hindered by natural obstacles and worsened by degenerative changes (19,20). The obstacles are superior articular process (SAP), cranial part of lower pedicle, osteophytes from posterior vertebral body, inferior articular process (IAP) and iliac crest for L5-S1 disc space (30) (Figure 1).
Depending upon the extent of migration, disc herniation is classified into 2 grades (19,20,31) (Figure 2).
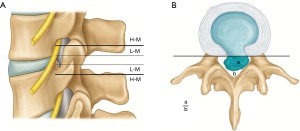
High grade migration
If the extent of migration is greater than the posterior disc height at the same disc level on T2 weighted sagittal MRI.
Low grade migration
If the extent of migration is lesser than posterior disc height at the same disc level on T2 weighted sagittal MRI, canal compromise is classified depending upon the extent of canal occupied by herniated disc on T2 weighted axial MRI (22).
- Mild: herniation less than 50% of the canal cross sectional area.
- Severe: herniation more than 50% of the canal cross sectional area (Figure 3).
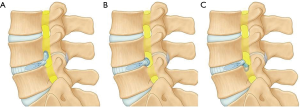
Author has described 3 anatomical route for transforaminal endoscopy (22). It has certain advantages over ‘outside-in’ technique as it can be applied for a wider range of lumbar disc.
Herniation including very difficult types of disc herniations can be tackled by ‘mobile outside-in’ technique due to its versatility of approach towards the target fragment.
- Intervertebral route for central, paracentral and high canal compromise LDH;
- Foraminal route for foraminal, superiorly migrated and far lateral LDH and;
- Suprapedicular route for inferiorly migrated LDH (Figure 4).
Materials and methods
Surgical procedure
The procedure is done under local anaesthesia or conscious sedation, in prone position on a radiolucent table with lumbar spine in slight flexion over Wilson’s frame. Advantage of Wilson’s frame is that it obliterates lumbar lordosis and increases antero-posterior dimensions of foramen to facilitate the safe passage of working cannula (Figure 5). We prefer to use arthropump with pressure set at 20–40 mm of Hg with 100% flow rate. The pressure can be adjusted according to clarity of endoscopic field. We do not use intra operative neuro monitoring (as advised by Prof. Yeung in his international meetings and symposium on endoscopic spine surgery) (32).
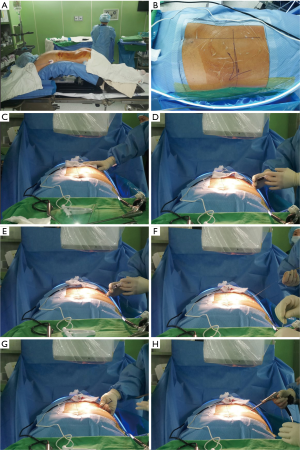
Following lines are marked with a marker pen under an image intensifier guidance (Figure 6).
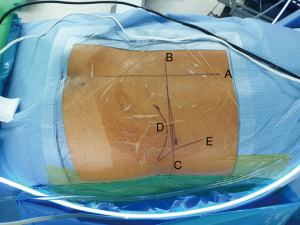
- Midline of the spine;
- Mid discal line in both anterio-posterior and lateral view and;
- Extent of the iliac crest.
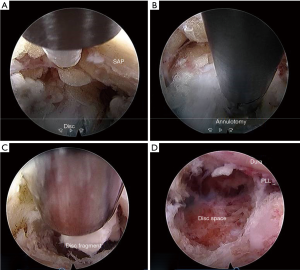
The borderline of back muscle and abdominal muscle checked using manual back assessment method (22) and skin entry point is marked along the mid discal line just medial to this borderline. discography performed by injecting 2 mL of solution containing 0.8% indigo carmine (Carmine, Korea United pharmaceutical, Yoenki, Korea) radiopaque dye (Iobrix Injection, Taejoon Pharma, Seoul, Korea) and normal saline in (2:1:2) ratio. Guide wire is inserted into the disc space through spinal needle and then needle is removed. Over the inserted guide wire blunt tip obturator is passed into the Kambin’s triangle followed by working cannula and endoscope. We use working channel with outer diameter of 7.5 mm and bevelled tip, the endoscope has 30° viewing angle, outer diameter of 6.5 mm, working channel diameter of 3.7 mm and working length 208 mm. TESSYSR (Joimax GmbH, Germany). Epidural fat and soft tissues i.e., intraforaminal ligaments are first structures to get noticed in the Kambin’s triangle after introduction of working channel into foramen. After clearing the soft tissue blue stained disc is easily identified in endoscopic field.
‘Mobile outside-in’ technique is carried out in 2 steps. In the first step, intra-discal decompression performed by transforsminal discectomy similar to the ‘inside-out’ technique. Annular fenestration is performed with the help of radiofrequency coagulator and disc is entered under endoscopic vision. Bevel type of cannula is inserted into the annular fenestration with bevel tip into annulus. Working channel levered downwards to achieve a ‘half-and-half’ view in which the dorsal half shows posterior longitudinal ligament, epidural space, dura and traversing nerve root; while ventral half shows annulus and disc fragment ventral to the posterior longitudinal ligament.
‘Mobile outside-in’ technique performed after decompressing the protruded disc sufficiently, trajectory of working channel and endoscope directed towards symptomatic migrated disc fragment called as ‘target fragmentectomy’. Depending on trajectory in the foramen transforaminal approach divided by an author further into:
- Suprapedicular route: inferiorly migrated disc;
- Intervertebral route: high canal compromised disc and foraminal route-superiorly migrated disc.
Flexible blunt tip probe or disc grasping forceps are used to expel a disc fragments from narrow epidural space of the spinal canal or foramen into the endoscopic working zone. Later it is removed with the help of rigid disc forceps.
Adequacy of decompression checked by observing the free floating dural sac, traversing and exiting nerve root in the epidural space by rotating the working channel and endoscope (Figure 7).
Results
In our case study of 184 consecutive patients with unilateral lower limb radiculopathy due to LDH operated with mobile outside-in technique we operated total 190 levels (L1–2: 4, L2–3: 17, L3–4: 27, L4–5: 123 and L5–S1: 19). The mean follow up period of the study was 19 months. we operated all types of disc herniations (14 central, 74 paracentral, 28 foraminal, 13 far lateral, 8 superior migration, 38 inferior migration and 9 high canal compromise) results were better than inside-out as well as outside-in technique compared with other parallel studies. the average VAS score for leg improved from 7.5 to 1.7; average ODI improved from 70% to 23%; 179 patients (97.3%) had satisfactory results according to MacNab’s criteria. patient with neurological deficit improved completely. we experienced 7.89% recurrence rate (15 patients) out of which 11 patients underwent repeat PETLD while 4 patients opted for open discectomy (Figures 8,9).
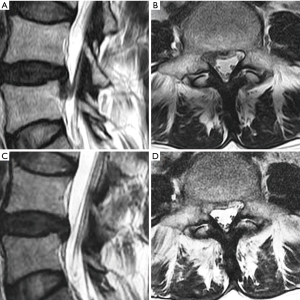
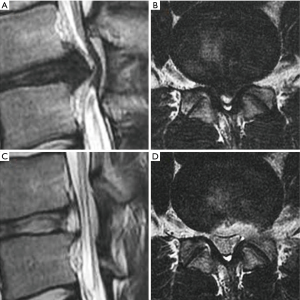
Discussion
Endoscopic transforaminal approach
Depending upon how pathology is approached endoscopic transforaminal approach is classified into:
- Inside-out technique;
- Outside-in technique and;
- Mobile outside-in technique.
Inside-out technique
The original ‘inside-out’ technique described by Yeung (14) with YESS based on the principle of in vivo visualization of pain generator inside the foramen by provocative method (14,33). It is purely intradiscal technique where initial docking of working cannula was inside disc space. Visualization of disc tissue is carried out in the disc cavity created by displacing disc fragment outside into the epidural space. Therefore initially cavity and the endoscopic instruments for working channel and decompression is very limited. The technique emphasized on indirect decompression of epidural space in order to preserve its vasculature and avoided post-operative scarring.
If needed, the working system can be gradually withdrawn from the disc into the epidural space of foramen to inspect any pathology in the foramen. Due to its pure intradiscal operative technique YESS technique has very limited indications in endoscopic spine surgery.
Indications of ‘inside-out’ technique
Internal disc disruption;
- Annular tears;
- Contained disc protrusions;
- Foraminal disc herniations;
- Extraforaminal disc herniations;
- Foraminal and extraforaminal stenosis;
- Failed back syndrome due to foraminal fibrosis and adhesions;
- Discitis.
Limitations
For extradiscal pathology ‘inside-out’ technique may be more destructive as it removes normal tissue more than pathological tissues;
- Not suitable for low and high grade migrated disc herniation;
- Not suitable for central protruded disc with high canal compromise.
Outside-in technique
Dr. Hoogland (15-18) introduced ‘outside-in technique’ as modification of inside-out technique. He named it as transforaminal/Thomas endoscopic spine surgery system (TESSYS/THESSYS). This technique was based on principles of serial dilation of intervertebral foramen by dilators, trephines or cannulated reamers over a guide wire to reach difficult part of spinal canals. It is called as expansile foraminoplasty by Hoogland. Whole foraminoplasty procedure is carried out meticulously under image intensifier guidance to ultimately land working cannula into safe zone of Kambin’s triangle. Sometimes over enthusiastic removal of bony structures can lead to post-operative pain and segmental instability; however introduction of the endoscopic drills has made a procedure safer. TESSYS technique certainly has an advantage over YESS technique as it can be applied for a wider range of LDH like extruded disc, Sequestrated disc, migrated disc and calcified disc and it has replaced YESS technique in last few years.
The ‘extreme lateral approach’ was introduced by Ruetten (34), as a variation to ‘outside-in’ technique for the direct epidural space using a rigid endoscope. It approached the target point from a far lateral distance from the midline to take a good exposure of the epidural space. Therefore, it had inevitably a low approach angle. Though outside in technique has expanded the pathologies which can be treated with transforaminal approach it comes with significant morbidity due to removal of bony structure to enhance visualization.
Indications of ‘outside in’ technique
In addition to pathologies can be treated by of ‘inside-out’ technique; ‘outside-in’ technique applicable for following indications:
- Huge central protruded disc: with high canal compromise;
- Sequestrated disc with low grade migration: superior migration, inferior migration;
- Recurred disc;
- After open lumbar discectomy;
- After percutaneous endoscopic lumbar discectomy.
- Calcified disc;
- Lateral recess stenosis;
- Multi-level herniated disc.
Limitations
Sequestrated disc with high grade migration particularly superior migration;
- Removes significant bone stalk;
- Chances of fracture in osteoporotic bone.
‘Mobile outside-in’ technique
‘Outside-in’ technique has covered number of indications that can be managed with transforaminal approach; still certain conditions cannot be treated with TESSYS technique such as high migration LDH and high canal compromise LDH. It requires gradual expansion of foramen towards disc migration with endoscopic drills which may compromise the stability of segment. ‘Mobile outside-in’ technique based on principle of precise placement of working channel into foramen and then carefully guiding the movement of working channel towards migrated fragment under endoscopic vision. With this manoeuvre we can minimise the amount of unnecessary bone resection in the foramen.
‘Mobile outside-in’ technique carried out in 2 steps
- Initial ‘outside-in’ technique: in 1st step, working channel is docked into Kambin’s triangle just like routine outside in technique in all cases. Intradiscal decompression done with half and half view. It gives indirect decompression of epidural space like inside-out technique.
- Targeted fragmentectomy: in 2nd step, working channel is slightly withdrawn and guided towards migrated disc fragment in the epidural space for fragmentectomy which provides direct decompression of epidural space like outside -in technique.
Depending on the direction of working channel in epidural space transforaminal approach further classified into:
- Intervertebral route: working channel is levered dorsally into canal to remove central or paracentral LDH with high canal compromise.
- Foraminal route: working channel directed superiorly towards exiting nerve root to remove foraminal, extra foraminal and superior migrated LDH.
- Suprapedicular route: working channel directed inferiorly towards inferior pedicle to remove inferior migrated LDH (22).
With the help of mobile outside-in technique all parts of spinal canal and foramen can be reached with minimal damage to normal bony elements and discal tissue. Special types of instruments like articulated and flexible retractors and forceps can decrease the difficulty level of procedure and improve learning curve for beginners; however it can be done with regular endoscopic spine instruments once a surgeon becomes familiar to the technique.
Indications
In addition to pathologies can be treated by of inside-out technique; outside-in technique applicable for high grade migration LDH.
Limitations
Relative limitations of transforaminal approach like:
- L5-S1 disc herniation;
- High iliac crest;
- Degenerative scoliosis.
Review of literature
In 2002, Yeung and Tsou (33) in their 307 consecutive patients of unilateral radiculopathy having LDH treated with inside out technique achieved 92.7% patients’ satisfaction rate with recurrence rate of 4.6%. Mean follow-up period was 19 months and complication rate was 3.9%. Hoogland (18) treated 262 patients with recurrence after microscopic or endoscopic discectomy by his outside in technique with significant improvement in VAS score of leg and back (69% and 66%). He noticed complication rate of 1.1% and reoperation rate of 7%. This study showed significant role of transforaminal approach in recurrent type of disc.
In 2007, Tzaan et al. (35) and Lee et al. (19) in their 2 different studies at 2 different centres achieved 89% satisfactory rate to inside out technique with Lee et al. having surprisingly zero percentage of complication and recurrence rate. Recently, Jasper et al. (36) applied transforaminal ‘outside-in technique for treating geriatric patients of degenerative lumbar disease (n=50, mean follow up: 19 months) with 74.6% excellent and good results on Macnab’s criteria and zero complication rate. Lewandrowski (37) in his case study of 220 patients with the longest follow up period of 46 months noticed 85% satisfaction rate with zero complication rate. Results of our case study with ‘mobile outside-in’ technique were comparable to recent studies. Recurrence rate in our study is comparable to other recent studies which was 5–18% (38-40). Recently, many studies have shown promising results; which is may be due to the development of better instrumentation, improved surgical skills and techniques.
The potential complications in ‘inside-out’ and ‘outside-in’ technique documented in literature which consist of injury to the exiting or traversing nerve root, incidental durotomy, injury to major vessels (41-47). most common post-operative complication experienced in the transforaminal approach is transient dysesthesia (5–15%) may be due to intra operative irritation of DRG or exiting nerve root, post-operative irritation due to small hematoma or re-herniation. though we have experienced zero complication rate, possibilities of potential complications during mobile outside in technique cannot be neglected (Table 2).
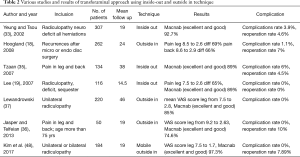
Full table
Future directions
In 2012, Osman (49) first utilized the transforaminal approach with expansile foraminoplasty for the interbody fusion in the treatment of degenerative lumbar conditions with almost 90% fusion rate. Recently, Mongestern et al. (50), Lee et al. (51) and Lewandrowski et al. (52) published their outcomes with different fusion rates. Interbody fusion via transforaminal approach has certain limitations such as inadequate foraminoplasty could make cage insertion difficult and also the iliac crest can act as an obstacle for the insertion of an interbody cage into the L5–S1 disc space. Further improvement in the instrumentation and interbody fusion devices will make the transforaminal approach suitable for the lumbar interbody fusion. However long term follow up for the fusion rate is warranted.
Conclusions
Endoscopic spine surgery has evolved dramatically in the last 30 years with development of new improved endoscopic optics and instrumentation expanding the indications of transforaminal endoscopic spine surgery. It also has given surgeons more flexibility to try and adapt a new technique with minimal damage and maximal conservation of normal anatomy of the spine. The ‘mobile outside-in’ technique described here has the advantages of both ‘inside-out’ and ‘outside-in’ technique. It is equally safe as ‘inside-out’ technique and provides careful handling of structures like extruded fragments avoiding neural structures; at the same time it is equally versatile as ‘outside-in’ technique in managing different types of disc herniations. The technique has longer and steep learning curve which demands patience in beginners for picking up this technique.
Acknowledgments
We would like to acknowledge scientific team members Ms. Jae Eun Park and Mr. Kyeong Rae Kim for providing assistance in acquiring full text articles and managing digital works.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.11.05). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim M, Kim HS, Kapoor A, et al. Feasibility of Full Endoscopic Spine Surgery in Patients Over the Age of 70 Years With Degenerative Lumbar Spine Disease. Neurospine 2019;16:6-14. [Crossref] [PubMed]
- Wang JC, Kim HS. Endoscopic Spinal Surgery (ESS): Prepare for a Happy 100-Year-Old! Neurospine 2019;16:4-5. [Crossref] [PubMed]
- Noordby E, Fraser R, Javid M, et al. Spine update chemonucleolysis. Spine 1996;21:1102-5. [Crossref] [PubMed]
- Poynton AR, Ofarrell DA, Mulcahy D, et al. Chymopapain chemonucleolysis: a review of 105 cases. J R Coll Surg Edinb 1998;43:407-9. [PubMed]
- Smith L. Enzyme dissolution of the nucleus pulposus in humans. JAMA 1964;187:137-40. [Crossref] [PubMed]
- Hijikata S. Percutaneous nuclectomy: A method of percutaneous nuclear extraction. J Toden Hospital 1975;5:39-44.
- Onik G, Helms C, Ginsburg L, et al. Percutaneous lumbar discectomy using a new aspiration probe. AJNR 1985;6:290-3.
- Kambin P, Brager MD. Percutaneous posterolateral discectomy. Anatomy and mechanism. Clin Orthop Relat Res 1987.145-54. [PubMed]
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med 1991;58:159-64. [PubMed]
- Kambin P. Percutaneous lumbar discectomy (Triangular Working Zone): Current practice. Surgical Rounds in Orthopaedics 1988:31-5.
- Mirkovic SR, Schwartz DG, Glazier KD. Anatomic considerations in posterolateral procedures. Spine (Phila Pa 1976) 1995;20:1965-71. [Crossref] [PubMed]
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999;21:39-42. [Crossref] [PubMed]
- Yeung AT. Minimally invasive disc surgery with the yeung endoscopic spine system (YESS). Surgical Technology International 1999;8:267-77. [PubMed]
- Hoogland T. Transforaminal endoscopic discectomy with forminoplasty for lumbar disc herniation. Surg Tech Orthop 2003;1-6.
- Hoogland T, Scheckenbach C. Die endoskopische transfor- aminale Diskektomie bei lumbalen Bandscheibenvorfällen. Orthop Prax 1998;34:352-5.
- Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine 2006;31:E890-7. [Crossref] [PubMed]
- Hoogland T, van den Brekel-Dijkstra K, Schubert M, et al. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: a prospective, cohort evaluation of 262 consecutive cases. Spine 2008;33:973-8. [Crossref] [PubMed]
- Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 2007;16:431-7. [Crossref] [PubMed]
- Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine (Phila Pa 1976) 2008;33:E508-15. [Crossref] [PubMed]
- Ruetten S, Komp M, Godolias G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: Prospective 2-year results of 331 patients. Minim Invasive Neurosurg 2006;49:80-7. [Crossref] [PubMed]
- Kim HS, Yudoyono F, Paudel B, et al. Analysis of clinical results of three different routes of percutaneous endoscopic transforaminal lumbar discectomy for lumbar herniated disk. World Neurosurgery 2017;103:442-8. [Crossref] [PubMed]
- Kambin P. Arthroscopic microdiscectomy. In: Kambin PR, editor. Minimal Intervention in Spinal Surgery. Baltimore: Urban & Schwarzenberg, 1991:67-1100.
- Kambin P, Gellman H. Percutaneous lateral discectomy of the lumbar spine: a preliminary report. ClinOrthop 1983.127-32.
- Hermantin FU, Peters T, Quartararo L, et al. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 1999;81:958-65. [Crossref] [PubMed]
- Kim HS, Ju CI, Kim SW, et al. Endoscopic transforaminal suprapedicular approach in high grade inferior migrated lumbar disc herniation. Journal of Korean Neurosurgical Society 2009;45:67. [Crossref] [PubMed]
- Chae KH, Ju CI, Lee SM, et al. Strategies for Noncontained Lumbar Disc Herniation by an Endoscopic Approach: Transforaminal Suprapedicular Approach, Semi-Rigid Flexible Curved Probe, and 3-Dimensional Reconstruction CT with Discogram. J Korean Neurosurg Soc 2009;46:312-6. [Crossref] [PubMed]
- Min JH, Kang SH, Lee JB, et al. Morphometric analysis of the working zone for endoscopic lumbar discectomy. J Spinal Disord Tech 2005;18:132-5. [Crossref] [PubMed]
- Lee SH, Kang B, Ahn Y, et al. Operative failure of percutaneous endoscopic lumbar discectomy: a radiologic analysis of 55 cases. Spine 2006;31:E285-90. [Crossref] [PubMed]
- Kim M, Kim HS, Oh SW, et al. Evolution of Spinal Endoscopic Surgery. Neurospine 2019;16:6-14. [Crossref] [PubMed]
- Ahn Y, Lee SH, Park WM, et al. Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg 2003;99:320-3. [PubMed]
- Yeung AT. Intra-operative Neuromonitoring in Percutaneous Transforaminal Surgery MIS Techniques and Results. Available online: http://www.isass.org/abstracts/isass12_regular_posters/isass12-453-Intra-operative-Neuromonitoring-in-Percutaneous-Transforaminal-Surgery.html
- Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002;27:722-31. [Crossref] [PubMed]
- Ruetten S, Komp M, Godolias G. An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine 2005;30:2570-8. [Crossref] [PubMed]
- Tzaan WC. Transforaminal percutaneous endoscopic lumbar discectomy. Chang Gung Medical Journal 2007;30:226. [PubMed]
- Jasper GP, Francisco GM, Telfeian AE. A retrospective evaluation of the clinical success of transforaminal endoscopic discectomy with foraminotomy in geriatric patients. Pain Physician 2013;16:225-9. [PubMed]
- Lewandrowski KU. “Outside-in” Technique, Clinical Results, and Indications with Transforaminal Lumbar Endoscopic Surgery: a Retrospective Study on 220 Patients on Applied Radiographic Classification of Foraminal Spinal Stenosis. Int J Spine Surg 2014;8:26. [Crossref] [PubMed]
- Weir BK, Jacobs G. Reoperation rate following lumbar discectomy. An analysis of 662 lumbar discectomies. Spine 1980;5:366-70. [Crossref] [PubMed]
- Österman H, Sund R, Seitsalo S, et al. Risk of multiple reoperations after lumbar discectomy: a population-based study. Spine 2003;28:621-7. [Crossref] [PubMed]
- Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Operative Orthopadie und Traumatologie 2005;17:641-61. [Crossref] [PubMed]
- Gore S, Yeung A. The “inside out” transforaminal technique to treat lumbar spinal pain in an awake and aware patient under local anesthesia: results and a review of the literature. Int J Spine Surg 2014.8. [PubMed]
- Sairyo K, Sakai T, Higashino K, et al. Complications of endoscopic lumbar decompression surgery. Minimally Invasive Neurosurgery 2010;53:175-8. [Crossref] [PubMed]
- Kim JE, Kim KH. Piriformis syndrome after percutaneous endoscopic lumbar discectomy via the posterolateral approach. Eur Spine J 2011;20:1663-8. [Crossref] [PubMed]
- Kaushal M, Sen R. Posterior endoscopic discectomy: Results in 300 patients. Indian Journal Of Orthopaedics 2012;46:81-5. [Crossref] [PubMed]
- Martín-Láez R, Martinez-Agueros JA, et al. Complications of Endoscopic Microdiscectomy Using the EASYGO! System: Is There Any Difference With Conventional Discectomy During the Learning-Curve Period? Acta Neurochir (Wien) 2012;154:1023-32. [Crossref] [PubMed]
- Ebata S, Sato H, Orii H, et al. Risk management in posterior spinal endoscopic surgery in lumbar diseases. J Orthop Sci 2013;18:369-73. [Crossref] [PubMed]
- Hsu HT, Chang SJ, Yang SS, et al. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013;22:727-33. [Crossref] [PubMed]
- Kim HS, Adsul N, Kapoor A, et al. A Mobile Outside-in Technique of Transforaminal Lumbar Endoscopy for Lumbar Disc Herniations. J Vis Exp 2018. [Crossref] [PubMed]
- Osman SG. Endoscopic transforaminal decompression, interbody fusion, and percutaneous pedicle screw implantation of the lumbar spine: A case series report. Int J Spine Surg 2012;6:157-66. [Crossref] [PubMed]
- Morgenstern R, Morgenstern C. Percutaneous Transforaminal lumbar interbody fusion (pTLIF) with a posterolateral approach for the treatment of denegerative disk disease: feasibility and preliminary results. Int J Spine Surg 2015;9:41. [Crossref] [PubMed]
- Lee SH, Erken HY, Bae J. Percutaneous transforaminal endoscopic lumbar interbody fusion: clinical and radiological results of mean 46-month follow-up. Biomed Res Int 2017;2017:3731983.
- Lewandrowski KU, Ransom NA, Ramírez León JF, et al. The Concept for A Standalone Lordotic Endoscopic Wedge Lumbar Interbody Fusion: The LEW-LIF. Neurospine 2019;16:82-95. [Crossref] [PubMed]


