
Patient selection protocols for endoscopic transforaminal, interlaminar, and translaminar decompression of lumbar spinal stenosis
Introduction
Spinal stenosis is one of the most frequent indications for surgery in the lumbar spine (1-13). With the increase in life expectancy and changes in societal expectation of higher functioning, on the whole, spine surgery is performed at a much higher rate and advanced age alone is no longer a contraindication for surgery (7,8). Risk factors with surgery in the lumbar spine in the elderly are similar to those in younger patients with the majority of unintended postoperative hospital readmission taking place because of poorly managed medical comorbidities rather than surgical site problems (5,6,11-13). Endoscopic spine surgery is an attractive alternative to open surgery because it is associated with a much lower risk of dural tears, nerve injuries, postoperative cardiopulmonary problems, and complication rates when compared to those reported with traditional open translaminar surgery (14,15).
Over the last ten years, endoscopic spine surgical techniques (ESST) have gained significant traction around the world with numerous publications coming out of hotspots in Asia reporting on the implementation of a variety of technology innovations and clinical protocols intended to facilitate ease of use with the endoscopic spinal decompression surgery and improve its associated clinical outcomes (16,17). Some of the most relevant advantages of ESST over other types of minimally invasive translaminar procedures are:
(I) Minimal bleeding and better viewing due to constant irrigation with physiological saline at pressures above 40 mmHg thereby maintaining venous compression;
(II) Direct and magnified visualization of the spinal anatomy at a much greater detail through a large visual field of view;
(III) The ability to simultaneously test and treat common pain generators within a spinal motion segment at an exceptional ability to access the different compartments of the lumbar spine (i.e., intradiscal- and epidural space) with greater mobility when compared to traditional translaminar microsurgery using the microscope without having to excessively retract nerve roots is unprecedented and unique to spinal endoscopy.
While these advantages of endoscopic spine surgery are widely accepted, and a myriad of clinical outcome studies suggest favorable clinical results in the majority of patients with success rates being reported in the 60% to 90% range (14,18-39), patient selection criteria for the different ESST approaches (transforaminal or interlaminar) are less well defined as they largely depend on the available equipment resources and more importantly on the skill level of the operating surgeon. This problem is particularly evident when surgery at the L5/S1 level is considered. Anatomical considerations such as a high-riding ilium or obliterated lateral access to the L5/S1 neuroforamen due to a hypertrophied superior or inferior articular process, or sacral alar may impact preoperative planning for the most suitable access to the painful compressive pathology. Additional problems may arise from transitional anatomy or a small or absent interlaminar window. A low pelvic incidence or a high sacral slope may make access to the intervertebral disc space difficult as well. Moreover, many times, the natural aging of the lumbar spine obliterates landmarks and distorts the otherwise familiar normal anatomy. In those patients, the operating surgeon may find the additional use of a tubular retractor system commonly used during translaminar microsurgery a useful aid during the endoscopic decompression. A hybrid endoscopy/tubular retractor or an endoscopically assisted tubular retractor surgery may be an additional consideration, particularly when attempting a more complex endoscopic decompression fusion surgery requiring an expanded foraminoplasty or involving placement of an interbody fusion cage. Employing well-thought-out algorithm stratifying patients preoperatively for the most suitable endoscopic or endoscopically-assisted decompression technique in the authors’ opinion has the potential to achieve favorable clinical ESST outcomes with higher consistency—a consideration that perhaps is of relevance to the novice endoscopic spine surgeon.
Therefore, this study aimed at testing an image-based endoscopic approach algorithm (EAA) consisting of four types to suggest a preferred endoscopic approach. Patient-specific criteria taken into consideration include the extent and location of the symptomatic compressive pathology, as well as access constraints dictated by the patient’s anatomy at the surgical level. The authors analyzed clinical outcomes with the application of this EAA over five years by only including patients with a minimum two-year follow-up using Oswestry Disability Index (ODI) (40), Visual Analogue Scale (VAS) (41), and modified Macnab criteria (42) as the primary clinical outcome measures.
Methods
Patients
This study included patients that underwent spinal stenosis surgery for symptomatic claudication leg between from January 2013 to February 2019. A total of 249 patients were selected for this analysis. There were 137 (55%) men and 112 (45%) women. Their age was 56.03±16.8 years ranging from 18 to 90. Quantile-quantile plot (Q-Q plot) showed normal age distribution among patients of this study (Figure 1). The minimum follow-up requirement of two years could be met by most patients. The average follow-up was 38.27±27.906 months ranging from 8 months to 10 years. All patients signed an informed consent form prior to surgery and before including them in this study.
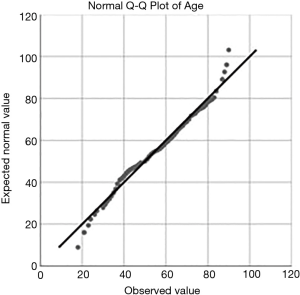
The quantile-quantile plot of the endoscopy patients’ age shows normal distribution. The average age was 56.03±16.8 years ranging from 18 to 90 years.
Inclusion/exclusion criteria
Patients were included in this study if the following criteria were used:
- Symptomatic lumbar radiculopathy, dysesthesias, or decreased motor function;
- Lumbar magnetic resonance imaging (MRI) showing central, foraminal, lateral recess or extraforaminal stenosis;
- Unrelenting pain, in spite of physical therapy, non-steroidal anti-inflammatories (NSAIDs), and transforaminal epidural steroid injections (TESI) for a minimum 8 weeks.
The following exclusion criteria were employed:
- Metastatic disease;
- Infection;
- Acute disc herniation;
- Patients who had surgical procedures on the cervical or lumbar spine, or other pain management procedures such as implantation of pain stimulators.
Patient selection protocol & surgical approach
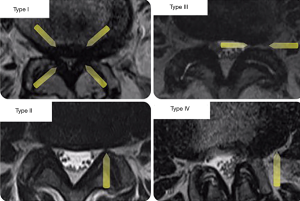
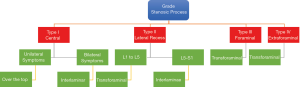
An algorithm was designed classifying patients into four groups according to the anatomic location and extent of stenosis, as illustrated by MRI imaging examples shown in Figure 2: type I—central canal stenosis (less than 100 mm2 cross-sectional area) (43), type II—lateral recess stenosis, type III—foraminal stenosis, and type IV—extraforaminal stenosis (3), they were subdivided according to the level of the lumbar spine that was compromised from L1 to L5 (Figure 2) (44). Patients with instability, obliquity of the facet joints, and those requiring surgery at the L5/S1 level were given additional considerations. The preoperative decision making algorithm is shown in Figure 3. The following surgical techniques were employed in this study.
Endoscopically assisted posterior decompression
In cases of central spinal, a tubular retractor system measuring 13 mm in diameter was placed through a small skin incision over the posterior elements of the surgical level after serial dilatators of the surgical corridors. This retractor system was also used for the percutaneous application of transpedicular screws in patients with concomitant spondylolisthesis. The individual steps were similar to traditional translaminar decompression and involved a proximal hemilaminectomy, a complete facetectomy, a distal hemilaminectomy, additional dissection of ligamentum flavum to achieve decompression of the central and lateral canal to expose the traversing and exiting nerve roots finally. This was followed by an over-the-top decompression of the contralateral recess in patients with bilateral symptoms. The authors preferred handheld manipulation of the tubular retractor system rather than a fixed position with a retractor arm on the predominantly symptomatic side. Bilateral skin incisions and decompression maneuvers were rarely necessary.
Transforaminal percutaneous endoscopic lumbar discectomy
The transforaminal decompression was performed under conscious sedation and local anesthesia. The authors place an endoscopic working cannular after serial dilation onto the lateral aspect of the foramen and perform a foraminoplasty and an outside-in decompression popularized by Hoogland (37,45,46) under direct visualization that has been described elsewhere (20,22,23,26). It is crucial to directly visualize and release the exiting and transversing roots. The authors prefer to perform the transforaminal surgery in the prone position and lumbar flexion using a 1.5-cm incision through which they employ a 7 mm working cannula to accommodate the 20º endoscope. The endoscopic decompression includes removal of the pars interarticularis rostrally, the facet joint complex, and the super articular process distally up to the pedicle. These decompression maneuvers would be of particular importance if an interbody fusion were also planned in patients with spondylolisthesis described below (47).
Transforaminal percutaneous endoscopic lumbar fusion
Patients requiring fusion and endoscopically assisted interbody fusion procedure was performed (47). After the aforementioned endoscopic decompression procedures, the vertebral endplates were decorticated and prepared using a 4-mm round drill bit. Rongeurs were used to extract loose disc fragments. A bipolar radiofrequency probe was used to control bleeding from epidural veins. Prior to placement of the cage and bone graft, the fusion bed was irrigated with physiological saline and antibiotics. Cancellous bone allograft was impacted into the intervertebral space anterior and lateral to the anticipated position of the fusion cage, which was typically placed over a nitinol guidewire under biplanar fluoroscopic guidance.
Interlaminar approach
During the interlaminar approach, originally popularized by Ruetten et al. (19,25,29,48), surgical access is created with the patient in the prone position, under conscious sedation. The skin incision is made as nearly medial in the craniocaudal middle of the interlaminar window as possible. A dilator, 6.9 mm in outer diameter, is inserted bluntly to the lateral edge of the interlaminar window. An operating sheath, with 7.9 mm outer diameter and beveled opening, is directed toward the ligamentum flavum. After that, the procedure is performed under visual control and constant irrigation, with a 25º rod lens endoscope with a 4.2 mm working channel measuring 165 mm in length.
Outcome & statistical analysis
Primary clinical outcomes measures for patients of this study were the modified Macnab criteria at the final follow-up (49). Also, pre- and postoperative VAS (41) and 40 scores were obtained. Statistical tests employed in the outcome analysis of this study included paired two-tailed t-test, and two-way cross-tabulation statistics to measure any statistically significant association between variables using IBM SPSS Statistics software, Version 25.0. Pearson Chi-Square and Fisher’s Exact test were employed to assess the strength of association between variables statistically. The mean, range, and standard deviation, and percentages of all nominal variables were calculated.
Results
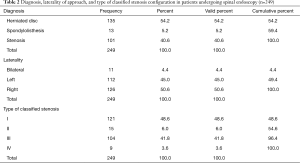
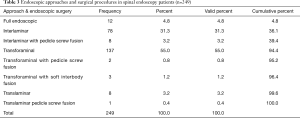
Analysis of the level distribution shows that L4/5 (115/249; 46.2%) and L5/S1 (83/249; 33.3%) followed by the L3/4 level (27/249; 10.8%) were the most commonly operated level. The remaining levels were operated on at a much lower frequency (Table 1). The majority of patients had surgery for herniated disc (135 patients). Another 101 patients were treated for spinal stenosis and another 13 for spondylolisthesis. The latter patients were treated with endoscopically assisted interbody and non-segmental fusion with an interbody fusion cage, bone graft, and percutaneous pedicle screws (Table 2). There were no major complications, such as hematomas, deep venous thrombosis, pulmonary embolus, infections, dural tears, graft extrusion, or neurological deficits. The fusion patients went on to have a successful clinical outcome with radiographic evidence of fusion. There were three patients suffering from recurrent disc herniations. They were ultimately treated with a revision endoscopic transforaminal discectomy surgery.

Full table
Full table
There was no statistically significant difference between left- (112/249; 45%) versus right-sided (126/249; 50.6%) approach. Another 11 (4.4%) patients had bilateral surgery. Regardless of the presence of associated stenosis or spondylolisthesis, patients’ disc herniations were graded as central in 129 of the 249 (51.8%), and as paracentral in the remaining 120 patients (48.2%). Thirteen patients also had associated spondylolisthesis (5.2%). The grading analysis of the stenosis configuration in the symptomatic surgical spinal motion segment showed that central canal stenosis (type I) was the most common scenario. It was the reason for surgical decompression in 121 (48.6%) patients. Foraminal stenosis (type III) was second most common scenario and the reason for endoscopic decompression in 104 patients (41.8%). Lateral recess (15/249; 6%), and extraforaminal stenosis (9/249; 3.6%) were by far less common reasons for endoscopic surgery.
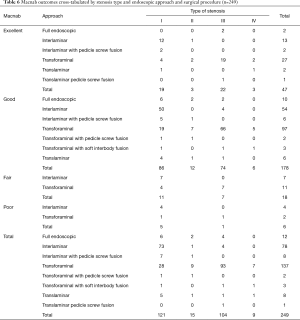
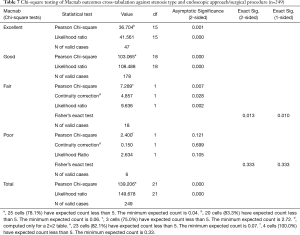
The most commonly used endoscopic approach was the transforaminal approach (137/249; 55.0%) followed by the interlaminar approach (78/249; 31.3%), the full endoscopic approach (12/249; 4.8%), and by the endoscopically assisted translaminar approach (8/249; 3.2%). If one includes the full-endoscopic decompressions and transforaminal fusion surgeries, the transforaminal approach was employed in 61% of all patients of this study (Table 3). Most of the fusion patients treated with pedicle screws (8/249; 3.2%) were endoscopically treated with the interlaminar approach. The remaining six endoscopically assisted procedures are listed in Table 3. At minimum follow-up and using the Macnab criteria, Excellent results were obtained in 47 patients (18.9%), Good in 178 (71.5%), Fair in 18 (7.2%) and Poor in 6 (2.4%) respectively (Table 4). The mean preoperative VAS was 7.9±1.5. and was reduced to 2.4±1.6 at final follow-up. The mean preoperative ODI was 49.1±17.5 and was reduced to 12.0±9.2 at final follow-up. Paired two-tailed t-test showed statistically significant VAS (P<0.0001) and ODI (P<0.0001) reductions as a result of the endoscopic surgery treatments (Table 5). Cross-tabulation of the Macnab outcomes versus the endoscopic approach and surgical technique using the stenosis grading by type is summarized in Table 6. Chi-square testing confirmed that the choice of endoscopic approach according to the proposed stenosis grading algorithm was associated with Excellent (Pearson Chi-square P=0.001) and Good (Pearson Chi-square P<0.0001) clinical outcomes according to modified Macnab criteria in statistically significant manner (Table 7). Even in the minimal number patients (11/249) with Fair—still improved—outcomes the choice of chosen approach was still associated with anatomic location of the stenotic process at a statistically significant level (Pearson Chi-square P=0.007).
Full table

Full table

Full table
Full table
Full table
Discussion
Patient selection for the endoscopic spinal surgery is of utmost importance (38,50,51). Understanding the pain generator is the key to obtaining favorable clinical outcomes (51). The indications for surgery are defined by unrelenting radiculopathy and neurogenic claudication symptoms that do not respond to non-operative medical care, physical therapy, and NSAIDs. Spinal injections are also often employed and are in some cases required by insurance carriers before authorizing surgery. The radiologists—willingly or not—have found themselves in the middle of the medical necessity discussion that was created by the insurance industry to determine whether proposed lumbar spine surgery is a covered benefit for the unaware patient seeking treatment (21,52). In the authors opinion, this development has somewhat distorted the role of advanced imaging studies in preoperative decision making. Nowadays, the MRI scan is often used as the “holy grail” of spine care discounting patient and physician input and other objective findings arising from history and physical examination, and other diagnostic test of higher prognostic value (22) than advanced cross-sectional imaging whose reporting of the surgical endoscopic anatomy often lacks detail. This has prompted the authors of this study to reevaluate the routine day-to-day use of the lumbar MRI scan in endoscopic spine surgery practice to overcome the dichotomy left by insufficient reporting of the clinically relevant stenotic lesions and the need to endoscopically treat compressive pathology often confined to a small area under the facet joint or in the lateral recess. This diagnostic gap was shown to affect up to thirty percent of patients complaining of the sciatica-type low back, and leg pain which did not meet traditional medical necessity criteria based on MRI reporting yet underwent successful endoscopic decompression (20). Therefore, the authors decided to formally analyze the benefit of an image-based patient stratification protocol they serendipitously employed over the years in their endoscopic spine practice. This protocol focused on determining the best endoscopic approach to a symptomatic stenotic process in the lumbar spine to aid the surgeon in obtaining clinical improvements with the endoscopic surgery reliably.
For this purpose, patients were stratified into four types of spinal stenosis assigning them to one predominant category which the authors thought correlated best with the primary pain generator corroborated by diagnostic injections as well as the patients’ history and physical examination. Stratifying patients based on the authors’ extensive clinical experience of successful clinical outcomes to one of these four stenosis types was predominantly based on MRI and CT cross-sectional imaging. The intent was to formalize a preoperative decision-making algorithm that would suggest to the endoscopic spine surgeon the most preferred approach and surgical technique based on ease of use to relieve the patient’s symptoms. It goes without saying that the surgeon’s technical abilities primarily drive clinical outcomes with endoscopic spinal surgery. Only endoscopic surgeons with the best skills will be able to reliably obtain clinical results by continuously adhering to the highest diagnostics standards. Given the diagnostic gap in the lumbar MRI scan, the authors wanted to correlate the choice of endoscopic approach with the stenosis type and the associated clinical outcomes.
Results of this study showed that favorable clinical outcomes could be obtained with the endoscopic decompression and endoscopically assisted fusion surgery in the vast majority of patients. Only 7% of the study patients did not have Excellent and Good clinical outcomes according to the modified Macnab criteria. However, 93% of them did (Table 4). This was supported by statistically significant reductions of the VAS and ODI scores as well (Table 5). The success rate of this study is approximately 10% higher than reported with most spine endoscopic outcome studies (14-17,20,22,23,26,31,38,50,51,53-55). The results of crosstabulation and chi-square statistical analysis of the chosen surgical approach and procedure with the type of stenosis and the correlated clinical outcomes showed a statistically significant association with successful resolution of the patients’ symptoms (Tables 6 and 7). However, attributing these successful outcomes solely to the choice of endoscopic approach would be an oversimplification of the diagnostic workup necessary to identify the primary pain generator preoperatively, and intraoperatively during direct visualization of the painful pathoanatomy within and outside the diseased intervertebral disc in the awake yet sedated patient who is asked during surgery to verbalize familiar or concordant pain during the videoendoscopic examination. The choice of a preferred approach to the painful compressive pathology may improve access to surgical anatomy—a consideration particularly relevant to the novice endoscopic spine surgeon—but it cannot substitute the required attention to detail in working up the painful pathoanatomy. In other words, employing the four-type approach selection protocol (Figure 2) is not a guarantee of successful clinical outcomes with the endoscopic surgery. It merely positions the surgeon to obtain most favorable access and not be limited by obstruction due to variation (transitional) or distortion of normal anatomy by hypertrophic degeneration of the facet joints, vertical collapse, and osteophytes.
The choice of the endoscopic approach may seem a trivial problem on the surface. In fact, 61% of all endoscopic and endoscopically assisted surgeries involved the transforaminal approach. It is the workhorse approach of spinal endoscopy and works well for most levels above the L5/S1 motion segment. At L5/S1, several anatomical considerations may dictate the use of additional approaches other than the transforaminal approach. What stands out though that the interlaminar approach was almost exclusively used in the treatment of type I—or central stenosis (Table 6) while there were many applications of the transforaminal approach in all four stenosis types including central stenosis. It was clearly the favorite endoscopic surgical approach employed by the two endoscopic spine surgeons who contributed their cases to this analysis because of its versatility. In the opinion of this team of authors, the transforaminal approach empowers the skilled endoscopic spine surgeon to deal with the most common painful pathoanatomical scenarios. Exceptions to this rule exist, and combination approaches are sometimes the best solution. They are encompassed in the four-zone stenosis protocol proposed by the authors.
Conclusions
The proposed four-zone stenosis protocol may aid the endoscopic spine surgeon in selecting the preferred endoscopic approach to the painful lumbar spine pathology. Selecting the best approach may facilitate achieving the goals of the endoscopic surgery. It is evident that additional comparative studies should examine the prognostic value of choosing the endoscopic approach based on the proposed four-type stenosis protocol by correlating its impact on outcomes with preoperative diagnostic injections and direct intraoperative visualization of symptomatic pain generators under local anesthesia and sedation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no direct (employment, stock ownership, grants, patents), or indirect conflicts of interest (honoraria, consultancies to sponsoring organizations, mutual fund ownership, paid expert testimony). The authors are not currently affiliated with or under any consulting agreement with any vendor that the clinical research data conclusion could directly enrich. This manuscript is not meant for or intended to endorse any products or push any other agenda other than to report the associated clinical outcomes with use of endoscopy versus laser. The motive for compiling this clinically relevant information is by no means created and/or correlated to directly enrich anyone due to its publication. This publication was intended to substantiate contemporary endoscopic spinal surgery concepts to facilitate technology advancements.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. IRB approval was obtained for this study (CEIFUS 106-19). Written informed consent was obtained from the patient for publication of this Original Study and any accompanying images.
References
- Hermansen E, Myklebust TA, Austevoll IM, et al. Clinical outcome after surgery for lumbar spinal stenosis in patients with insignificant lower extremity pain. A prospective cohort study from the Norwegian registry for spine surgery. BMC Musculoskelet Disord 2019;20:36. [Crossref] [PubMed]
- Held U, Steurer J, Pichierri G, et al. What is the treatment effect of surgery compared with nonoperative treatment in patients with lumbar spinal stenosis at 1-year follow-up? J Neurosurg Spine 2019;5:1-9. [PubMed]
- Raad M, Donaldson CJ, El Dafrawy MH, et al. Trends in isolated lumbar spinal stenosis surgery among working US adults aged 40-64 years, 2010-2014. J Neurosurg Spine 2018;29:169-75. [Crossref] [PubMed]
- Paulsen RT, Bouknaitir JB, Fruensgaard S, et al. Prognostic Factors for Satisfaction After Decompression Surgery for Lumbar Spinal Stenosis. Neurosurgery 2018;82:645-51. [Crossref] [PubMed]
- Held U, Burgstaller JM, Wertli MM, et al. Prognostic function to estimate the probability of meaningful clinical improvement after surgery - Results of a prospective multicenter observational cohort study on patients with lumbar spinal stenosis. PLoS One 2018;13:e0207126. [Crossref] [PubMed]
- Caruso R, Pesce A, Martines V, et al. Assessing the real benefits of surgery for degenerative lumbar spinal stenosis without instability and spondylolisthesis: a single surgeon experience with a mean 8-year follow-up. J Orthop Traumatol 2018;19:6. [Crossref] [PubMed]
- Imajo Y, Taguchi T, Neo M, et al. Complications of spinal surgery for elderly patients with lumbar spinal stenosis in a super-aging country: An analysis of 8033 patients. J Orthop Sci 2017;22:10-5. [Crossref] [PubMed]
- Antoniadis A, Ulrich NH, Schmid S, et al. Decompression surgery for lumbar spinal canal stenosis in octogenarians; a single center experience of 121 consecutive patients. Br J Neurosurg 2017;31:67-71. [Crossref] [PubMed]
- Giannadakis C, Solheim O, Jakola AS, et al. Surgery for Lumbar Spinal Stenosis in Individuals Aged 80 and Older: A Multicenter Observational Study. J Am Geriatr Soc 2016;64:2011-8. [Crossref] [PubMed]
- Försth P, Olafsson G, Carlsson T, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Modhia U, Takemoto S, Braid-Forbes MJ, et al. Readmission rates after decompression surgery in patients with lumbar spinal stenosis among Medicare beneficiaries. Spine (Phila Pa 1976) 2013;38:591-6. [Crossref] [PubMed]
- Försth P, Michaelsson K, Sanden B. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis?: A two-year follow-up study involving 5390 patients. Bone Joint J 2013;95-B:960-5. [Crossref] [PubMed]
- Javalkar V, Cardenas R, Tawfik TA, et al. Reoperations after surgery for lumbar spinal stenosis. World Neurosurg 2011;75:737-42. [Crossref] [PubMed]
- Lewandrowski KU. Incidence, Management, and Cost of Complications After Transforaminal Endoscopic Decompression Surgery for Lumbar Foraminal and Lateral Recess Stenosis: A Value Proposition for Outpatient Ambulatory Surgery. Int J Spine Surg 2019;13:53-67. [Crossref] [PubMed]
- Lewandrowski KU. Readmissions After Outpatient Transforaminal Decompression for Lumbar Foraminal and Lateral Recess Stenosis. Int J Spine Surg 2018;12:342-51. [Crossref] [PubMed]
- Choi G, Pophale CS, Patel B, et al. Endoscopic Spine Surgery. J Korean Neurosurg Soc 2017;60:485-97. [Crossref] [PubMed]
- Choi G, Prada N, Modi HN, et al. Percutaneous endoscopic lumbar herniectomy for high-grade down-migrated L4-L5 disc through an L5-S1 interlaminar approach: a technical note. Minim Invasive Neurosurg 2010;53:147-52. [Crossref] [PubMed]
- Yeung A, Roberts A, Zhu L, et al. Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine 2019;16:52-62. [Crossref] [PubMed]
- Wasinpongwanich K, Pongpirul K, Myat Lwin KM, et al. Full-Endoscopic Interlaminar Lumbar Discectomy: Retrospective Review of Clinical Results and Complications in 545 International Patients. World Neurosurg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin Neurol Neurosurg 2019;179:74-80. [Crossref] [PubMed]
- Yeung A, Kotheeranurak V. Transforaminal Endoscopic Decompression of the Lumbar Spine for Stable Isthmic Spondylolisthesis as the Least Invasive Surgical Treatment Using the YESS Surgery Technique. Int J Spine Surg 2018;12:408-14. [Crossref] [PubMed]
- Lewandrowski KU. Successful outcome after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis: The positive predictive value of diagnostic epidural steroid injection. Clin Neurol Neurosurg 2018;173:38-45. [Crossref] [PubMed]
- Lewandrowski KU. Endoscopic Transforaminal and Lateral Recess Decompression After Previous Spinal Surgery. Int J Spine Surg 2018;12:98-111. [Crossref] [PubMed]
- Liu C, Zhou Y. Percutaneous Endoscopic Lumbar Diskectomy and Minimally Invasive Transforaminal Lumbar Interbody Fusion for Recurrent Lumbar Disk Herniation. World Neurosurg 2017;98:14-20. [Crossref] [PubMed]
- Markovic M, Zivkovic N, Spaic M, et al. Full-endoscopic interlaminar operations in lumbar compressive lesions surgery: prospective study of 350 patients. "Endos" study. J Neurosurg Sci 2016. [Epub ahead of print]. [PubMed]
- Lewandrowski KU. "Outside-in" technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg 2014. [Crossref] [PubMed]
- Komp M, Hahn P, Ozdemir S, et al. Operation of lumbar zygoapophyseal joint cysts using a full-endoscopic interlaminar and transforaminal approach: prospective 2-year results of 74 patients. Surg Innov 2014;21:605-14. [Crossref] [PubMed]
- Gore S, Yeung A. The "inside out" transforaminal technique to treat lumbar spinal pain in an awake and aware patient under local anesthesia: results and a review of the literature. Int J Spine Surg 2014. [Crossref] [PubMed]
- Ruetten S, Komp M, Hahn P, Oezdemir S. Decompression of lumbar lateral spinal stenosis: full-endoscopic, interlaminar technique. Oper Orthop Traumatol 2013;25:31-46. [Crossref] [PubMed]
- Birkenmaier C, Komp M, Leu HF, et al. The current state of endoscopic disc surgery: review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician 2013;16:335-44. [PubMed]
- Yeung AT, Gore S. In-vivo Endoscopic Visualization of Patho-anatomy in Symptomatic Degenerative Conditions of the Lumbar Spine II: Intradiscal, Foraminal, and Central Canal Decompression. Surg Technol Int 2011;21:299-319. [PubMed]
- Komp M, Hahn P, Merk H, et al. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011;24:281-7. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech 2009;22:122-9. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop 2009;33:1677-82. [Crossref] [PubMed]
- Kuonsongtum V, Paiboonsirijit S, Kesornsak W, et al. Result of full endoscopic uniportal lumbar discectomy: preliminary report. J Med Assoc Thai 2009;92:776-80. [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9. [Crossref] [PubMed]
- Hoogland T, van den Brekel-Dijkstra K, Schubert M, et al. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: a prospective, cohort evaluation of 262 consecutive cases. Spine (Phila Pa 1976) 2008;33:973-8. [Crossref] [PubMed]
- Yeung AT, Yeung CA. Minimally invasive techniques for the management of lumbar disc herniation. Orthop Clin North Am 2007;38:363-72. abstract vi. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg 2007;50:219-26. [Crossref] [PubMed]
- Erçalık T, Gencer Atalay K, Sanal Toprak C, et al. Outcome measurement in patients with low back pain undergoing epidural steroid injection. Turk J Phys Med Rehabil 2019;65:154-9. [Crossref] [PubMed]
- Reed CC, Wolf WA, Cotton CC, et al. A visual analogue scale and a Likert scale are simple and responsive tools for assessing dysphagia in eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;45:1443-8. [Crossref] [PubMed]
- Macnab I. The surgery of lumbar disc degeneration. Surg Annu 1976;8:447-80. [PubMed]
- Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am 2003;34:281-95. [Crossref] [PubMed]
- Schroeder GD, Kurd MF, Vaccaro AR. Lumbar Spinal Stenosis: How Is It Classified? J Am Acad Orthop Surg 2016;24:843-52. [Crossref] [PubMed]
- Hoogland T. Percutaneous endoscopic discectomy. J Neurosurg 1993;79:967-8. [PubMed]
- Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol 2005;17:641-61. [Crossref] [PubMed]
- Lewandrowski KU, Ransom NA, Ramirez Leon JF, et al. The Concept for A Standalone Lordotic Endoscopic Wedge Lumbar Interbody Fusion: The LEW-LIF. Neurospine 2019;16:82-95. [Crossref] [PubMed]
- Ruetten S, Hahn P, Oezdemir S, et al. Full-endoscopic uniportal decompression in disc herniations and stenosis of the thoracic spine using the interlaminar, extraforaminal, or transthoracic retropleural approach. J Neurosurg Spine 2018;29:157-68. [Crossref] [PubMed]
- Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am 1971;53:891-903. [Crossref] [PubMed]
- Yeung AT, Yeung CA. Advances in endoscopic disc and spine surgery: foraminal approach. Surg Technol Int 2003;11:255-63. [PubMed]
- Yeung AT, Yeung CA. In-vivo endoscopic visualization of patho-anatomy in painful degenerative conditions of the lumbar spine. Surg Technol Int 2006;15:243-56. [PubMed]
- Yeung AT, Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin Neurol Neurosurg 2019;181:52. [Crossref] [PubMed]
- Tsou PM, Alan Yeung C, Yeung AT. Posterolateral transforaminal selective endoscopic discectomy and thermal annuloplasty for chronic lumbar discogenic pain: a minimal access visualized intradiscal surgical procedure. Spine J 2004;4:564-73. [Crossref] [PubMed]
- Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976) 2002;27:722-31. [Crossref] [PubMed]
- Tsou PM, Yeung AT. Transforaminal endoscopic decompression for radiculopathy secondary to intracanal noncontained lumbar disc herniations: outcome and technique. Spine J 2002;2:41-8. [Crossref] [PubMed]


