
The evolution of interlaminar endoscopic spine surgery
Introduction
Degenerative lumbar spine disorder is a common and significant cause of disability in the world (1). With the trend of an aging population, degenerative spine disorders lead to an increase of the global burden and a rise of the need for spinal care (2). The concepts of functional preservation and enhancing post-operative recovery have been general consensus in modern spinal care. Therefore, the field of minimally invasive spine surgery has progressed significantly during the past two decades. The advancement of image modalities provides accurate preoperative radiological evaluation and plan. Moreover, with the development of surgical technologies, minimally invasive spine surgery has been prevalent gradually due to minimize damage to non-pathogenic structures, durable effectiveness with less complication rates, and shorter hospital stay (3).
As for minimally invasive spine surgery, full-endoscopic spine surgery has been a viable option in recent decades. The full-endoscopic surgery is performed with the application of an endoscope that consists of a 20–30-degree rod-lens camera system with a light source, a working channel and an irrigation channel. The design of endoscope provides clear visual control during operation and minimize traumatization to normal soft tissue with uni-portal tract. In comparison to traditional surgery, endoscopic spine surgery provides advantages such as less soft tissue trauma, reduced blood loss, decreased damage to the epidural blood supply and consequent epidural fibrosis, shorter hospital stays, shorter time to return to work (4-8). Through the past decade, the advancement of endoscopic instruments, such as different designs of endoscope and endoscopic burrs or punches, has promoted the technical progress and extensive application of the full-endoscopic spine surgery.
The interlaminar and transforaminal endoscopic spine surgeries have been the representatives for the endoscopic spine surgery. Each technique has developed many modified skills in treating different pathologies. The interlaminar endoscopic spine surgery (IESS) was proposed after transforaminal technique but it further contributed to expand the indications of endoscopic spine surgery, especially in treating spinal stenosis. In this review, the authors will introduce the evolution of IESS following development of the three generations endoscope system (Figure 1) and discuss the application of IESS in the present and future.
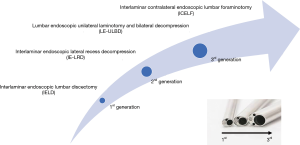
Evolution of IESS
In the early stage, full-endoscopic spine surgery was derived from percutaneous lumbar discectomy. Initially, percutaneous lumbar discectomy was performed under fluoroscopic guidance by posterolateral approach without direct visualization (9,10). In 1991, Kambin developed arthroscopic lumbar discectomy through a “triangular safe zone”, which was recognized as the prototype of modern transforaminal endoscopic lumbar discectomy (TELD) (11). During 1990, most endoscopic operations were performed through transforaminal access with uniportal scope under fluid flow (11-15). The preliminary study of TELD also showed similar efficacy and recurrence rate compared with conventional microdiscectomy in selected patients (5). However, TELD at the L5–S1 level is sometimes technically challenging due to anatomical limitation of surgical access through posterolateral approach (16). The anatomical studies revealed that the corridor through transforaminal approach to reach L5–S1 foramen may be limited by high iliac crest, smallest intertransverse space, and narrow foramen compared with other levels cranially (17,18). Therefore, interlaminar approach technique was introduced as an alternative solution for full-endoscopic discectomy at L5–S1.
First generation: interlaminar endoscopic lumbar discectomy (IELD)
In the lumbar spine, the interlaminar window at L5–S1 level was greatest and the width of the interlaminar space was maximum at 31 mm (19). The interlaminar approach for lumbar discectomy has been done with the aid of microscope since 1970s (20-22) and microendoscopic system since late 1990s (23-25). Spine surgeons have been familiar with this corridor by posterior approach to do lumbar spine surgeries. Therefore, IELD was initially considered to be an advanced form of microscopic lumbar discectomy. The IELD represented the discectomy performed with the full-endoscopic system through the interlaminar window. Patients could be in either the prone or the lateral decubitus position. The operation could be performed under conscious sedation, epidural or general anesthesia. The endoscope had an outer diameter of 6mm and working channel of 2.7 mm (YESS; Richard Wolf, GmbH, Knittlingen, Germany). Usually, the surgeon can insert the endoscope into epidural space for discectomy without doing laminotomy. While reaching epidural space, surgeons could remove herniated disc with aid of forceps or laser through working channel.
In 2006, two pilot studies of IELD for L5–S1 herniated intervertebral disc were reported from Korean and German groups respectively. Choi et al. reported 90.8% favorable result among 65 patients with more than 1.5 years follow-up after L5–S1 IELD in Korea (26). Patients were under local anesthesia during surgery and the mean hospital stay was 12 hours. Furthermore, a prospective study reported by Ruetten et al. in 2006 also showed a promising outcome in 331 patients who underwent IELD by the same endoscopic equipment and the recurrence rate was 2.4% with 2 years follow-up period (27).
There were two major technical differences between the two groups. First, Choi et al. split ligamentum flavum with sequential dilator under fluoroscopic guidance, but Ruetten et al. made an incision by 5 mm in the lateral ligamentum flavum under visual control. The ligamentum flavum has an important role in structural integrity and prevention of epidural scarring (28,29). However, the Choi’s technique cannot be performed under direct visualization. Thereafter, this ligamental preservation technique was modified by Kim et al. with serial dilators under direct visualization of endoscope (30). Second, the Choi’s technique included annular modulating procedure with circumferential coagulation of the radiofrequency probe and side firing laser probe. The annular sealing technique can help with the decrease of early recurrence after IELD (31). In a recent report, 96.25% of patients underwent IELD with both these structural preservation techniques could have favorable outcome (32).
In this stage, IELD was effective mainly in treating non-migrated or low-grade migrated soft disc at L5–S1 level. The first-generation endoscopic instruments could only remove soft tissue such as ligament and disc. The osseous diameter of the interlaminar window was the most important factor in assessing the feasibility of IELD. The previous radiological study revealed that the width of interlaminar window in the lower lumbosacral spine L4–5 and L5–S1 usually allows for performing IELD (33). However, lateral drilling is necessary for interlaminar discectomy at upper lumbar levels. Furthermore, there were problems with the small working channel in the endoscope and lack of durable instruments for resection of bone. That is, narrow interlaminar window results in limited indication for IELD with first generation endoscope system.
Second generation: interlaminar endoscopic discectomy with laminotomy
With the necessity of bony work in endoscopic spine surgery, second generation of endoscope with an outer diameter of 7.9 mm and a 4.2 mm working channel and instruments such as specially designed endoscopic burrs and punches were produced. In 2007, Ruetten et al. began to utilize the newly designed optics and instruments to eliminate the bony limitation under full-endoscopic visualization in some cases for removal of migrated disc (34). According to this report in 2007, they had to resect bone segment of the inferior articular process and cranial lamina for discectomy in 14% patients underwent IELD. There was no failure or conversion in the consecutive case series with both interlaminar and transforaminal approach. The large intra-endoscope working channel and tools suitable for laminotomy enabled to enlarge interlaminar window. Subsequent studies also revealed that the treatment of different levels and types of disc herniation was effective with IELD, included level of L4–5 and above.
Moreover, the second-generation endoscope also launched a new era of endoscopic spine surgery. Degenerative lumbar lateral recess stenosis is usually caused by hypertrophic facet joint, herniated disc, and ligamentous structures. Symptoms such as neurogenic claudication with radicular signs can occur in this situation. Microscopic laminectomy has been the main surgical therapy until now. However, with second generation endoscopic system, the technique of interlaminar endoscopic lateral recess decompression (IE-LRD) could also be effective with less tissue damage. In 2009, Ruetten et al. firstly reported prospective and randomized controlled study of surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach (7). He applied the second-generation endoscope and 4-mm burr to do lateral recess decompression through ipsilateral interlaminar approach. Decompression was accomplished by cranial and caudal laminotomy, and partial medial facetectomy with endoscopic burr or shaver to unroof unilateral lateral recess. Then, the surgeon could remove the ligamentum flavum with micro-punch and grasping forceps. The ipsilateral traversing root was completely decompressed in the similar fashion of the microsurgical procedure. The clinical outcome of the patients underwent endoscopic decompression was the same with the patients with microsurgical decompression. However, the rate of complications and revisions were significantly reduced in the endoscopic group. Patients could have less post-operative pain and rapid recovery to normal life with endoscopic surgery. Therefore, the technique of IE-LRD further expanded the indications of IESS.
Third generation: full-endoscopic laminotomy for bilateral decompression
The spinal stenosis in the lumbar spine is the most common degenerative disease that spine surgeons treat (35). It is defined as a reduced cross-sectional area of the vertebral canal. The pathogenesis may arise from serial changes of facet hypertrophy, ligamentous hypertrophy, intervertebral disc bulging, and osteophyte growth. In the perspective of surgical anatomy, there are three stenotic zone included central canal stenosis, lateral recess stenosis, and foraminal stenosis (36). Until now, the most commonly performed surgical treatment for treating lumbar stenosis has been open microscopic laminectomy (37,38). With the advent of endoscopic lumbar discectomy, it became a natural progression to apply the technique to decompress symptomatic spinal stenosis in minimally invasive way.
Although the transforaminal endoscopic decompression is feasible for foraminal or lateral recess decompression, this technique might be impracticable to decompress central canal stenosis (39,40). By contrast, the interlaminar endoscopic decompression is theoretically feasible to solve central canal stenosis and lateral recess stenosis simultaneously from posterior approach. Khoo et al. also reported similar concept of minimally invasive decompressive laminotomy with microendoscopic approach (41). However, it was time-consuming to use small-diameter endoscopic burrs for bilateral laminotomy with second-generation full-endoscopic system. Therefore, the third generation of endoscope with outer diameter of 9.5 mm and working channel diameter of 5.6mm was derived from the demand of the efficient laminotomy (Table 1).
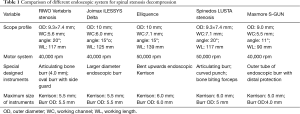
Full table
Komp et al. reported clinical results of 74 patients underwent the interlaminar endoscopic decompression for central spinal canal stenosis in 2011 (42). By using the third-generation endoscope system with burrs up to 5.5 mm in diameter, they developed the technique of lumbar endoscopic unilateral laminotomy and bilateral decompression (LE-ULBD) with removal of ligamentum flavum. First, the ipsilateral decompression was performed by means of cranial and caudal laminotomy, partial facetectomy, and flavum resection. Then, the contralateral decompression was done with over-the-top technique to undercut the contralateral lamina with high-speed burr (Figure 2). The mean operating time was 44 minutes (ranged from 35 to 61 minutes) in one segment. The results of LE-ULBD were comparable with conventional surgery but complication rates and revision rates were low. There was also no increased fusion rate in 2 years follow-up after LE-ULBD. Therefore, the LE-ULBD became a standard technique in endoscopic spine surgery for decompression of central canal stenosis.
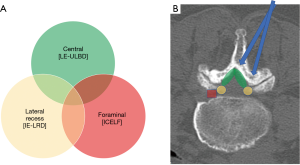
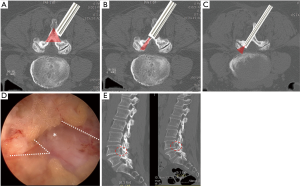
Sometimes, multiple stenotic zones are encountered in lumbar spinal stenosis (Figure 3). The common pathologies leading to lateral recess stenosis are hypertrophic facet joint, bulging of the disc annulus or posterior endplate osteophytes (43,44). These degenerative anatomical changes might also result in foraminal stenosis and symptomatic root entrapment at the same time. The intervertebral foramen is usually accessible by transforaminal endoscopic approach. However, the lateral recess decompression is challenging by transforaminal endoscopic route. Besides, the ipsilateral interlaminar endoscopic decompression occasionally requires extensive removal of the medial facet joint on the affected side to unroof the lateral recess and expose the medial pedicle surface, which may result in segmental instability. Therefore, the technique of interlaminar contralateral lumbar endoscopic foraminotomy (ICLEF) was proposed as an alternative to achieve unilateral decompression of the lateral recess and intervertebral foramen (45). By using the burr under direct endoscopic visualization, the base of the spinous process, caudal edge of the upper lamina, and rostral edge of the lower lamina are partially removed in medial-to-lateral direction from midline to contralateral side. The ventro-medial facetectomy and flavectomy are performed until the traversing and exiting nerve roots were decompressed (Figure 4). The clinical results by Hwang et al. showed that 14 patients with unilateral radiculopathy had significant improvement of pain and life of quality after contralateral interlaminar endoscopic decompression surgery and there was no dural tear, neurological injury, or revision. In another study by Kim et al., 24 of 26 patients had favorable outcome and only one patient needed revision surgery because of reduced disc height and grade I spondylolisthesis (46).
By using appropriate instruments, the interlaminar endoscopic decompression techniques can provide versatile application in treating all kinds of pathologies safely and effectively (Table 2). The third-generation endoscope and newly designed tools improved the efficiency of laminotomy. Through widening interlaminar window, the range of surgical accessibility can improve in transverse plane of the spinal canal. Therefore, surgeons can attain bilateral decompression via unilateral approach to solve central canal, lateral recess, and contralateral foraminal stenosis simultaneously. Besides, the endoscopic decompression techniques can minimize injuries to the facet joints and the posterior midline structures, which might have a beneficial effect on preserving spinal stability. Hence, the interlaminar endoscopic surgery has been widely accepted and utilized in the full-endoscopic lumbar spine surgeries.

Full table
Present application of interlaminar endoscopic techniques
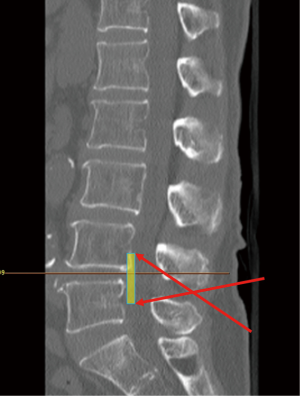
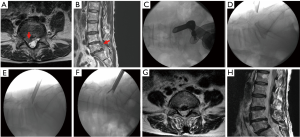
The mobility of interlaminar endoscopic technique provides broad indication in dealing with all kinds of patho-anatomies (Table 3). For surgical treatment of high-grade migrated disc herniation, patients underwent TELD had worse clinical and radiological outcomes than those with near-migrated discs and therefore open surgery should be considered (47,48). Although TELD with foraminoplasty by partial removal of ventral facet and/or pedicle could increase the width of the foramen and expose the anterior epidural space, IELD could reach the far-migrated fragment without destruction of facet joint or pedicle, which may cause subsequent instability. Through the interlaminar window, the cranio-caudal trajectory of the endoscope is flexible and can be designed according to migrated fragment evaluated on pre-operative images (Figure 5). For selected patient with high-grade down-migrated L4–5 disc herniation, L5–S1 IELD could also remove sequestrated disc through wide interlaminar window without bony destruction (49). In circumstances with overhang lamina, surgeons can utilize tailored laminotomy to widen the interlaminar window and switching different sizes of endoscope to reach far-migrated disc fragment (Figure 6).
Full table
The future of IESS
For the past decade, the advancement of IESS has focused on the neural decompression from various pathologies. Clinical evidence has accumulated rapidly and the full-endoscopic surgical techniques have been proven to be effective and safe for treatment of degenerative lumbar spondylopathy. IELD is continuously evolving and standardized. However, steep learning curve remains an obstacle to promote endoscopic spine surgery. Nowadays, the CT-based intraoperative navigation system is available and feasible in minimally invasive spine surgery, especially spinal instrumentation. The recent report indicated that the navigation improved the learning curve of full-endoscopic lumbar discectomy (50). Moreover, radiation exposure to the surgeon is also an important issue and adequate protection equipment such as lead apron is essential (51). The surgeons are free from radiation exposure and while doing surgeries assisted with navigation system (52). Therefore, navigation guided full-endoscopic spine surgery, such as LE-ULBD or full-endoscopic lumbar foraminotomy, will emerge to improve the learning curve and safety of spine endoscopic surgery.
Recently, some reports about applications of interlaminar endoscopic surgery in spinal stenosis or intervertebral disc herniation of thoracic spine have been reported (53-55). The application of IESS in the cervical spine is also emerging (56,57). With more clinical evidence reported, the indication of IESS may also include the thoracic and cervical spinal surgery in the future.
Conclusions
In terms of the IESS, the interlaminar window is like the door to all kinds of pathologies. The endoscopic surgical instruments, such as endoscopic burr, Kerrison, and endoscope with larger diameter, are the keys to open the interlaminar window. The improvement of endoscopic tools drives the evolution of the IESS by widening the interlaminar window. Furthermore, it also broadens the indications of the endoscopic lumbar spine surgery. In the future, comparison studies between different techniques of minimally invasive spine surgery and the application in thoracic and cervical spine surgeries may be the direction of future development.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.10.06). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Kim reports personal fees from Richard Wolf GmbH, personal fees from Elliquence, LLC, during the conduct of the study; Dr. Chen, Dr. Jabri, Dr. Lokanath, and Dr. Song have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968-74. [Crossref] [PubMed]
- Hurwitz EL, Randhawa K, Yu H, et al. The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J 2018;27:796-801. [Crossref] [PubMed]
- Kanno H, Aizawa T, Hahimoto K, et al. Minimally invasive discectomy for lumbar disc herniation: current concepts, surgical techniques, and outcomes. Int Orthop 2019;43:917-22. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9. [Crossref] [PubMed]
- Kim MJ, Lee SH, Jung ES, et al. Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol 2007;68:623-31. [Crossref] [PubMed]
- Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician 2015;18:61-70. [PubMed]
- Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine 2009;10:476-85. [Crossref] [PubMed]
- Lee DY, Shim CS, Ahn Y, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc 2009;46:515-21. [Crossref] [PubMed]
- Hijikata S. Percutaneous nucleotomy. A new concept technique and 12 years' experience. Clin Orthop Relat Res 1989.9-23. [Crossref] [PubMed]
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med 1991;58:159-64. [PubMed]
- Kambin P, O'Brien E, Zhou L, et al. Arthroscopic microdiscectomy and selective fragmentectomy. Clin Orthop Relat Res 1998.150-67. [PubMed]
- Mathews HH. Transforaminal endoscopic microdiscectomy. Neurosurg Clin N Am 1996;7:59-63. [Crossref] [PubMed]
- Savitz MH. Same-day microsurgical arthroscopic lateral-approach laser-assisted (SMALL) fluoroscopic discectomy. J Neurosurg 1994;80:1039-45. [Crossref] [PubMed]
- Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg 1993;78:216-25. [Crossref] [PubMed]
- Mirkovic SR, Schwartz DG, Glazier KD. Anatomic considerations in lumbar posterolateral percutaneous procedures. Spine (Phila Pa 1976) 1995;20:1965-71. [Crossref] [PubMed]
- Ebraheim NA, Xu R, Huntoon M, et al. Location of the extraforaminal lumbar nerve roots. An anatomic study. Clin Orthop Relat Res 1997.230-5. [Crossref] [PubMed]
- Reulen HJ, Muller A, Ebeling U. Microsurgical anatomy of the lateral approach to extraforaminal lumbar disc herniations. Neurosurgery 1996;39:345-50; discussion 350-1. [Crossref] [PubMed]
- Ebraheim NA, Miller RM, Xu R, et al. The location of the intervertebral lumbar disc on the posterior aspect of the spine. Surg Neurol 1997;48:232-6. [Crossref] [PubMed]
- Caspar W. editor. A New Surgical Procedure for Lumbar Disc Herniation Causing Less Tissue Damage Through a Microsurgical Approach. Berlin, Heidelberg: Springer Berlin Heidelberg, 1977.
- Goald HJ. Microlumbar discectomy: follow up of 147 patients. Spine (Phila Pa 1976) 1978;3:183-5. [Crossref] [PubMed]
- Wilson DH, Kenning J. Microsurgical lumbar discectomy: preliminary report of 83 consecutive cases. Neurosurgery 1979;4:137-40. [Crossref] [PubMed]
- Nakagawa H, Kamimura M, Uchiyama S, et al. Microendoscopic discectomy (MED) for lumbar disc prolapse. J Clin Neurosci 2003;10:231-5. [Crossref] [PubMed]
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002;51:S129-36. [Crossref] [PubMed]
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999;21:39-42. [Crossref] [PubMed]
- Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery 2006;58:ONS59-68; discussion ONS59-68.
- Ruetten S, Komp M, Godolias G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: prospective 2-year results of 331 patients. Minim Invasive Neurosurg 2006;49:80-7. [Crossref] [PubMed]
- Aydin Y, Ziyal IM, Duman H, et al. Clinical and radiological results of lumbar microdiskectomy technique with preserving of ligamentum flavum comparing to the standard microdiskectomy technique. Surg Neurol 2002;57:5-13; discussion 14. [Crossref] [PubMed]
- Ozer AF, Oktenoglu T, Sasani M, et al. Preserving the ligamentum flavum in lumbar discectomy: a new technique that prevents scar tissue formation in the first 6 months postsurgery. Neurosurgery 2006;59:ONS126-33; discussion ONS-33.
- Kim CH, Chung CK. Endoscopic interlaminar lumbar discectomy with splitting of the ligament flavum under visual control. J Spinal Disord Tech 2012;25:210-7. [Crossref] [PubMed]
- Kim HS, Park JY. Comparative assessment of different percutaneous endoscopic interlaminar lumbar discectomy (PEID) techniques. Pain Physician 2013;16:359-67. [PubMed]
- Lee JS, Kim HS, Jang JS, et al. Structural Preservation Percutaneous Endoscopic Lumbar Interlaminar Discectomy for L5-S1 Herniated Nucleus Pulposus. Biomed Res Int 2016;2016:6250247.
- Sakçı Z, Onen MR, Fidan E, et al. Radiologic Anatomy of the Lumbar Interlaminar Window and Surgical Considerations for Lumbar Interlaminar Endoscopic and Microsurgical Disc Surgery. World Neurosurg 2018;115:e22-6. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine 2007;6:521-30. [Crossref] [PubMed]
- Szpalski M, Gunzburg R. Lumbar spinal stenosis in the elderly: an overview. Eur Spine J 2003;12 Suppl 2:S170-5. [Crossref] [PubMed]
- Andreisek G, Imhof M, Wertli M, et al. A systematic review of semiquantitative and qualitative radiologic criteria for the diagnosis of lumbar spinal stenosis. AJR Am J Roentgenol 2013;201:W735-46. [Crossref] [PubMed]
- Surgeon J, Waddell G, Grant I. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev 2000;5:85-6.
- Ma XL, Zhao XW, Ma JX, et al. Effectiveness of surgery versus conservative treatment for lumbar spinal stenosis: A system review and meta-analysis of randomized controlled trials. Int J Surg 2017;44:329-38. [Crossref] [PubMed]
- Ahn Y, Keum HJ, Lee SG, et al. Transforaminal Endoscopic Decompression for Lumbar Lateral Recess Stenosis: An Advanced Surgical Technique and Clinical Outcomes. World Neurosurg 2019;125:e916-24. [Crossref] [PubMed]
- Lewandrowski KU. Incidence, Management, and Cost of Complications After Transforaminal Endoscopic Decompression Surgery for Lumbar Foraminal and Lateral Recess Stenosis: A Value Proposition for Outpatient Ambulatory Surgery. Int J Spine Surg 2019;13:53-67. [Crossref] [PubMed]
- Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery 2002;51:S146-54. [Crossref] [PubMed]
- Komp M, Hahn P, Merk H, et al. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011;24:281-7. [Crossref] [PubMed]
- Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Lumbar spinal nerve lateral entrapment. Clin Orthop Relat Res 1982.171-8. [PubMed]
- Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine (Phila Pa 1976) 1988;13:313-20. [Crossref] [PubMed]
- Hwang JH, Park WM, Park CW. Contralateral Interlaminar Keyhole Percutaneous Endoscopic Lumbar Surgery in Patients with Unilateral Radiculopathy. World Neurosurg 2017;101:33-41. [Crossref] [PubMed]
- Kim HS, Patel R, Paudel B, et al. Early Outcomes of Endoscopic Contralateral Foraminal and Lateral Recess Decompression via an Interlaminar Approach in Patients with Unilateral Radiculopathy from Unilateral Foraminal Stenosis. World Neurosurg 2017;108:763-73. [Crossref] [PubMed]
- Lee SH, Kang BU, Ahn Y, et al. Operative failure of percutaneous endoscopic lumbar discectomy: a radiologic analysis of 55 cases. Spine (Phila Pa 1976) 2006;31:E285-90. [Crossref] [PubMed]
- Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 2007;16:431-7. [Crossref] [PubMed]
- Choi G, Prada N, Modi HN, et al. Percutaneous endoscopic lumbar herniectomy for high-grade down-migrated L4-L5 disc through an L5-S1 interlaminar approach: a technical note. Minim Invasive Neurosurg 2010;53:147-52. [Crossref] [PubMed]
- Ao S, Wu J, Tang Y, et al. Percutaneous Endoscopic Lumbar Discectomy Assisted by O-Arm-Based Navigation Improves the Learning Curve. Biomed Res Int 2019;2019:6509409.
- Ahn Y, Kim CH, Lee JH, et al. Radiation exposure to the surgeon during percutaneous endoscopic lumbar discectomy: a prospective study. Spine (Phila Pa 1976) 2013;38:617-25. [Crossref] [PubMed]
- Pireau N, Cordemans V, Banse X, et al. Radiation dose reduction in thoracic and lumbar spine instrumentation using navigation based on an intraoperative cone beam CT imaging system: a prospective randomized clinical trial. Eur Spine J 2017;26:2818-27. [Crossref] [PubMed]
- Ruetten S, Hahn P, Oezdemir S, et al. Full-endoscopic uniportal decompression in disc herniations and stenosis of the thoracic spine using the interlaminar, extraforaminal, or transthoracic retropleural approach. J Neurosurg Spine 2018;29:157-68. [Crossref] [PubMed]
- Miao X, He D, Wu T, et al. Percutaneous Endoscopic Spine Minimally Invasive Technique for Decompression Therapy of Thoracic Myelopathy Caused by Ossification of the Ligamentum Flavum. World Neurosurg 2018;114:8-12. [Crossref] [PubMed]
- Hur JW, Kim JS, Seung JH. Full-endoscopic interlaminar discectomy for the treatment of a dorsal migrated thoracic disc herniation: Case report. Medicine (Baltimore) 2019;98:e15541. [Crossref] [PubMed]
- Kim CH, Kim KT, Chung CK, et al. Minimally invasive cervical foraminotomy and diskectomy for laterally located soft disk herniation. Eur Spine J 2015;24:3005-12. [Crossref] [PubMed]
- Zheng C, Huang X, Yu J, et al. Posterior Percutaneous Endoscopic Cervical Diskectomy: A Single-Center Experience of 252 Cases. World Neurosurg 2018;120:e63-7. [Crossref] [PubMed]


