
Endoscopic surgical treatment for symptomatic spinal metastases in long-term cancer survivors
Introduction
Approximately 1.7 million people develop cancer annually (1) and 5–10% of affected patients will develop symptomatic vertebral metastases (2,3), making the spine one the most common locations of disease spread. In fact, spinal regions are the most common osseous location of metastatic lesions (4) with studies demonstrating evidence of spinal metastases in 30–90% of patients who died of cancer (5) Most lesions spread to the thoracic spine representing 70% of cases, while 20% spread to the lumbar spine and 10% to the cervical spine (3,6,7). Spinal metastases present variably (8) but symptoms of axial bone pain and back pain are present in 85–95% of cases and can be caused by periosteal stretching, mechanical instability, or nerve root compression (5,9). Nerve root compression caused by metastatic lesions can also lead to disabling radicular pain as well as sensory or motor deficits in the corresponding myotomal and dermatomal distributions. These symptoms are often debilitating and can adversely impact quality of life, physical function, and psychosocial performance in cancer patients.
Despite its severity, recent medical advances in cancer treatment have significantly lengthened life expectancies in patients with spinal metastatic disease (4). Although not curative, surgical interventions can play a vital role in the overall management of cancer patients harboring spinal lesions by significantly improving quality of life and helping maintain functional independence. Goals of surgical treatment include preserving neurologic function, pain relief, removal of tumor mass, and spinal stabilization (5,8). However, cancer-associated comorbidities may preclude, or significantly increase, the perioperative risk associated with invasive open surgical interventions traditionally utilized to manage spinal metastatic disease. Furthermore, patients with end-stage disease may not want to endure the longer recovery period associated with these invasive procedures. Given the increased risks and longer recovery times associated with open procedures, combined with the realization that surgical intervention is typically palliative, minimally invasive spinal techniques have been increasingly evaluated in patients with spinal metastases. Minimally invasive interventions have been shown to have decreased complication rates, perioperative morbidity, blood loss, postoperative pain, duration of stay, length of hospitalization, and overall healthcare costs (10-19). Additionally, minimally invasive techniques allow patients to initiate radiation therapy and rehabilitation earlier. Endoscopic spine surgery is a minimally invasive technique that has been utilized in many degenerative spinal disorders but has not been well studied in patients with metastatic disease. We describe our experience and technique in 3 patients undergoing awake endoscopic transforaminal surgery for treatment of symptomatic spinal metastases.
Methods
This study is a retrospective chart review of 325 patients operated on by 1 surgeon between 2014 and 2018 with a minimum follow-up of one year. The study received ethics approval (approved by the Lifespan IRB #1194051-1.) The focus of this study is on the feasibility of offering awake transforaminal endoscopic spine surgery in patients with metastatic spine disease. We identified 3 patients in this cohort who suffered from neurologic symptoms related to their metastatic spine lesions and underwent endoscopic procedures.
Operative procedure
For the endoscopic (Joimax® TESSYS) spine procedures, the patient was positioned in the prone position on a Wilson frame with flexed hips and knees. The procedure was done under local anesthesia (1% lidocaine with epinephrine) and intravenous sedation; the level of anesthetic was titrated, so the patient was able to communicate with the surgeon throughout the procedure. Percutaneous entry was established through the skin between 5 and 12 cm lateral to the midline. Using intermittent fluoroscopic guidance, alternating between lateral and anterior-posterior (AP) view, a 15 cm 18-guage needle was advanced and placed at the superior endplate of the inferior vertebral body through Kambin’s triangle, between the exiting and traversing nerves. An AP fluoroscopic view was used to confirm the needle was at the medial border of the pedicle of the inferior vertebral body. A 6 mm incision was made over the needle, and a K-wire was placed in the needle, the needle removed, and sequential dilators placed over the K-wire. Sequential reamers were used to enlarge the neural foramen by removing the ventral aspect of the superior articulating process of the inferior vertebral body. At this point the beveled cannula tubular dilator was placed over the sequential dilators, the dilators removed, and the 7 mm outer diameter Joimax® rigid working channel endoscope channel was inserted through the tubular retractor. Under endoscopic visualization, endoscopic graspers were used to biopsy and debulk tumor and endoscopic drills and Kerrison rongeurs were used to remove bone and ligament to successfully decompress affected nerve roots.
Results
Case 1
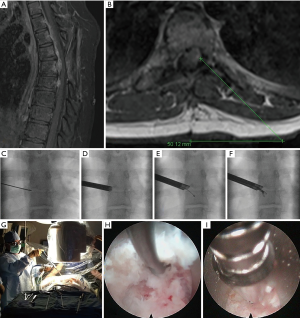
The patient is a 16-year-old female who originally presented with a larger T5-6 ventral epidural tumor. It was resected completely through a costotransversectomy approach. Initial pathologic diagnosis was only malignant tumor and it was treated with radiation when it started to recur one year later. Figure 1A shows the T2-T8 “string of grapes” appearing tumor in the ventral epidural space. The patient complained of severe left thoracic radicular pain in a T6 distribution. Figure 1A,B show the large compressive ventral epidural tumor behind the T6 vertebral body. Figure 1C,D,E,F show the AP fluoroscopic images to access the ventral epidural tumor through the T5-6 foramen. Figure 1G,H,I are photographs of the surgery (G) and endoscopic camera views from the tumor resection (H,I). The patient underwent a successful outpatient awake tumor biopsy and partial resection with immediate resolution of her thoracic radicular pain. Pathological analysis confirmed an Ewing’s-like tumor. She subsequently underwent chemotherapy and expired 2 years after her endoscopic surgery with brain and spine metastases.
Case 2
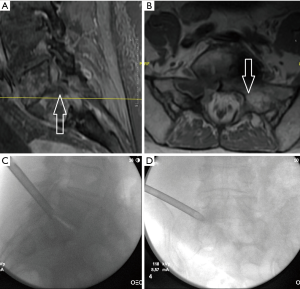
The patient is a 75-year-old female with stage IV non-small cell lung carcinoma, squamous histology of the lung with metastasis to scalp, spine, and lymph nodes. She had progressive radicular pain despite radiation to a metastatic lesion in her sacrum. Even though she was on long acting opioids for her pain, she required hospital admission to control the pain in her left leg. Figure 2A,B show her MRI and Figure 2C,D show the approach to her endoscopic tumor biopsy, resection and decompression of her left L5-S1 foramen. The surgery was done as an outpatient, and the patient survives 2 years after her endoscopic surgery without recurrence of her left leg pain.
Case 3
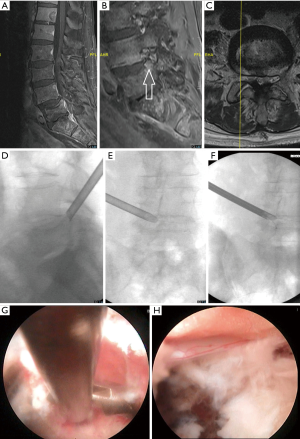
The patient is 76-year-old male with metastatic prostate cancer treated with multiple palliative radiation courses. He presents with MRI evidence of disease progression (Figure 3A) including metastatic disease throughout the thoracic, lumbar, sacral spine as well as bilateral iliac bones. He suffered from severe right L5 radiculopathy and foot drop despite comprehensive interventional pain management. His MRI demonstrated a right L5 vertebral endplate fracture extending into the L4-5 foramen (Figure 3B,C). Figure 3D,E,F show fluoroscopic images detailing the endoscopic approach to treating the fracture and Figure 3G,H show the endoscopic camera views of the tumor resection (G) and decompression of the L5 nerve root (H). He had significant improvement in his neurologic symptoms following his endoscopic procedure and was able to walk without an assistive device. He remained symptom free at his 1 year follow up.
Case 4
The patient is an 85-year-old male with metastatic prostate cancer treated with a prostatectomy, radiation, and chemotherapy. He presents with MRI evidence of metastatic disease to the spine and suffers from a severe left lumbar 3–4 radiculopathy despite interventional pain management. Figure 4A is a sagittal MR image that demonstrates the L3-4 foraminal compression secondary to the prostate metastasis. Figure 4B,C show fluoroscopic images that demonstrate the approach to the foraminal decompression. The patient had dramatic relief of his radicular symptoms after surgery and remained symptom free for his 1 year follow up.

Discussion
Recent advances in treatment have increased life expectancy for many different cancers, necessitating a reevaluation of surgical management strategies utilized for spinal metastases. Minimally invasive procedures, with fewer perioperative risks and quicker recovery times, present an attractive option for reducing symptom burden in cancer patients with vertebral involvement. In addition, cancer patients with poor prognoses often find the long recovery times required following invasive open surgery unpalatable (4). The shorter recovery period following minimally invasive procedures is often more desirable in patients with end-stage disease and short life expectancy.
Despite a variety of prognostic scoring systems, selecting patients with spinal metastases who would benefit from surgical intervention remains one of the most difficult aspects of cancer therapy. A number of schemata have been presented to guide management in this patient population. Tokuhashi et al. developed a system to assess the prognosis of metastatic spinal tumors based on general medical condition, the number of extraspinal bone metastases, the number of metastases in the vertebral body, metastases to the major internal organs, the primary site of the cancer, and the severity of spinal cord compression. They found each of these factors correlated with prognosis (20,21). Sioutos et al. developed a survival prediction tool based on the anatomic site of primary carcinoma, preoperative neurologic deficit, extent of disease, number of vertebral bodies involved, tumor location, and age (22). Tomita et al. proposed a scoring system based on only three factors including grade of malignancy, visceral metastases, and bone metastases (23). van der Linden et al. used the Karnofsky performance score, primary tumor site, and presence/absence of visceral metastases as the basis of their scoring system (24). The Bauer scoring system uses metastatic load, site of primary tumor, and presence of pathologic fracture (25). In 2008, Leithner et al. attempted an external comparison of these prognostic scoring systems, finding the Bauer and modified Bauer scores were practicable and highly predictive when compared to other systems. However, the authors acknowledge that no one prognostic score should be rigidly adhered to when deciding on a treatment plan (26,27). The “NOMS” framework, developed in 2013, takes into account the neurologic, oncologic, mechanical, and systemic factors of the patient’s disease to guide decision making. Each of these categories has its own sub-scoring system that takes into account the need for multidisciplinary care and allows for dynamic integration of multi-modal treatment options (28). Of note, regardless of the scoring system utilized, the most important prognostic indicator for surgery is the initial functional status (8,29). The large number of prognostic scoring systems and wide range of included variables demonstrate a clear need for external validation and meta-analysis to increase ease and accuracy when making surgical treatment choices.
Despite the abundance of variables used in predicting the prognosis from vertebral metastases, the majority of guidelines recommend that patients with life expectancies less than 3 months be treated non-surgically due to risks of perioperative morbidity (4,27,30). To assess if minimally invasive techniques could reduce this morbidity, Pennington et al. performed an analysis of nine studies between 2006–2018 directly comparing minimally invasive surgery with open surgery for the management of spinal metastases. These cases were a mix of retrospective case series, case control studies, and prospective case series. They found that minimally invasive surgery resulted in significantly less blood loss, shorter operative times, shorter hospital stays, and lower complication rates with similar rates of neurologic improvement and pain relief when compared to traditional open surgery (30). These findings were consistent with other studies that have demonstrated lower associated soft tissue damage (31,32), lower post-operative infection rates (33-37), lower post-operative pain, and shorter lengths of stay (38) from minimally invasive procedures while offering similar functional results to conventional surgery (31,39-42). While there is an abundance of evidence in favor of minimally invasive surgery, it is currently mostly low quality and the efficacy of these techniques compared to open, more invasive surgery must continue to be evaluated (30). However, overall minimally invasive techniques appear to be more desirable in end -stage patients with short life expectancies who have debilitating pain or neurologic symptoms.
A number of minimally invasive techniques have been described for use in the setting of symptomatic spinal metastases with the goal of tumor resection, spinal decompression, or spinal stabilization. A common thread between all of these minimally invasive procedures is that that they use smaller incisions and cause less muscle damage. The most frequently used minimally invasive techniques utilize tubular retractors to access affected spine regions so that biopsy, decompression or placement of instrumentation can be completed or placing spinal instrumentation through a percutaneous route. Posterolateral corpectomy, in which a minimally invasive approach is used to remove up to 80% of an afflicted vertebral body, has been validated as a safe and effective treatment for tumor removal by multiple studies (42-47), though these studies are all limited by small sample size. Zairi et al. prospectively evaluated ten patients with metastasis to the thoracolumbar spine and neurological compromise who underwent minimally invasive transpedicular vertebrectomy and spinal cord decompression through a tubular expandable retractor. They reported no complications and concluded that minimally invasive decompression of spine metastasis is a safe and effective palliative option (48). Vertebral stabilization can be achieved in patients with instability secondary to metastatic tumor involvement by percutaneous placement of pedicle screws, which involves placing unipedicular or bipedicular cannula into fractured vertebral bodies and injecting a quick setting polymethylmethacrylate cement to stabilize the vertebral body. While there are some reports of cement extravasation outside the vertebral body, this technique has demonstrated safety, efficacy, and cost-effectiveness (3,49). Balloon kyphoplasty is performed by drilling bilateral channels into a fractured vertebral body and placing inflatable balloon tamps inside. The balloons are then slowly inflated to improve the kyphotic angle, creating a cavity in the center of the vertebral body that is then filled with cement. This technique has been found to improve disability scores, increase quality of life, and decrease analgesic use compared to conservative treatment (50). The Kiva Treatment System consists of a flexible implant that provides mechanical support for the vertebral body and acts as a vessel to contain and direct flow of cement, all done with an implantation technique that allows for more consistent results than percutaneous vertebroplasty or balloon kyphoplasty. This procedure has been demonstrated to be safe and effective by Korovessis et al. and the KAST trial (49,51). Furthermore, radiofrequency ablation can be used for percutaneous, localized destruction of unresectable spinal metastatic tissue while sparing surrounding healthy tissue (3). Goetz et al. demonstrated significant reduction of pain and reduced opioid use with radiofrequency ablation (52) and Thanos et al. showed significant reductions in pain and analgesic use (53). Continuing the trend of multi-disciplinary minimally invasive approaches to cancer care, external beam radiation in conjunction with the aforementioned percutaneous procedures is now an option (54) including intraoperative radiotherapy during balloon kyphoplasty (55).
Endoscopic techniques have been increasingly utilized in a variety of degenerative spinal disorders but have rarely been described in patients with metastatic disease. The lack of need for general anesthesia, low associated complication rates, and short recovery times make endoscopic treatment of metastatic spine tumors an alluring option. Gao et al. described a case utilizing a percutaneous transforaminal endoscopic lumbar approach for palliative decompression of a 71-year-old woman with a symptomatic L3 vertebral body colon metastasis. The patient achieved prompt and permanent pain relief without complications until expiring 6 months after surgery (56). Tsai et al. reported the use of an interlaminar endoscopic approach for nerve decompression in an 80-year-old man with a hepatocellular metastatic lesion in the sacrum. Their patient had near complete resolution of his severe radicular pain and regained the ability to ambulate (57). Mclain described the use of an endoscope to assist in visualization of the spinal cord during the final stages of corpectomy completed in patients with metastatic spinal tumors (58). Our case series further supports the alternative use of percutaneous endoscopic procedures in the management of symptomatic spinal metastases.
As surgical management options for symptomatic spinal metastases advance, the goal should be to develop techniques that continue to reduce perioperative complications, promote quick recovery times, and effectively treat neurologic symptoms caused by tumor involvement. In our limited series, we have demonstrated the feasibility of minimally invasive awake transforaminal endoscopic surgery for treatment of radicular pain caused by nerve compression from metastatic spinal tumors. This also alleviates the need for hardware placement or cement injection, potentially reducing complication rates. As our patient population is small, continued evaluation of the efficacy of awake, endoscopic transforaminal surgery is required, including comparisons of success rates and risk profiles compared to other minimally invasive techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.10.14). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study received ethics approval (approved by the Lifespan IRB #1194051-1).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts and Figures 2019, 2019.
- Binning MJ, Gottfried ON, Klimo J, et al. Minimally invasive treatments for metastatic tumors of the spine. Neurosurg Clin N Am 2004;15:459-65. [Crossref] [PubMed]
- Stephenson MB, Glaenzer B, Malamis A. Percutaneous Minimally Invasive Techniques in the Treatment of Spinal Metastases. Curr Treat Options Oncol 2016;17:56. [Crossref] [PubMed]
- Barzilai O, McLaughlin L, Lis E, et al. Outcome analysis of surgery for symptomatic spinal metastases in long-term cancer survivors. J Neurosurg Spine 2019;26:1-6. [PubMed]
- Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease: A review. J Neurosurg Spine 2010;13:94-108. [Crossref] [PubMed]
- Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998;89:599-609. [Crossref] [PubMed]
- Stallmeyer MJ, Zoarski GH, Obuchowski AM. Optimizing Patient Selection in Percutaneous Vertebroplasty. J Vasc Interv Radiol 2003;14:683-96. [Crossref] [PubMed]
- Bhatt AD, Schuler JC, Boakye M, et al. Current and emerging concepts in non-invasive and minimally invasive management of spine metastasis. Cancer Treat Rev 2013;39:142-52. [Crossref] [PubMed]
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann Neurol 1978;3:40-51. [Crossref] [PubMed]
- Assaker R. Minimal access spinal technologies: state-of-the-art, indications, and techniques. Joint Bone Spine 2004;71:459-69. [Crossref] [PubMed]
- Holly LT, Schwender JD, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus 2006;20:E6. [Crossref] [PubMed]
- Hsieh PC, Koski TR, Sciubba DM, et al. Maximizing the potential of minimally invasive spine surgery in complex spinal disorders. Neurosurg Focus 2008;25:E19. [Crossref] [PubMed]
- Jakobs TF, Trumm C, Reiser M, et al. Percutaneous vertebroplasty in tumoral osteolysis. Eur Radiol 2007;17:2166-75. [Crossref] [PubMed]
- Kan P, Schmidt MH. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus 2008;25:E8. [Crossref] [PubMed]
- Kanter AS, Mummaneni PV. Minimally invasive spine surgery. Neurosurg Focus 2008;25:E1. [Crossref] [PubMed]
- Miscusi M, Polli FM, Forcato S, et al. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: surgical technique and early clinical results. J Neurosurg Spine 2015;22:518-25. [Crossref] [PubMed]
- Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. J Clin Neurosci 2011;18:741-9. [Crossref] [PubMed]
- Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus 2009;27:E9. [Crossref] [PubMed]
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine 1990;15:1-4. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110-3. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A Revised Scoring System for Preoperative Evaluation of Metastatic Spine Tumor Prognosis. Spine 2005;30:2186-91. [Crossref] [PubMed]
- Sioutos PJ, Arbit E, Meshulam CF, et al. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer 1995;76:1453-9. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine 2001;26:298-306. [Crossref] [PubMed]
- van der Linden YM, Dijkstra SP, Vonk EJA, et al. Prediction of survival in patients with metastases in the spinal column. Cancer 2005;103:320-8. [Crossref] [PubMed]
- Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand 1995;66:143-6. [Crossref] [PubMed]
- Bauer H, Tomita K, Kawahara N, et al. Surgical strategy for spinal metastases. Spine 2002;27:1124-6. [Crossref] [PubMed]
- Leithner A, Radl R, Gruger G, et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J 2008;17:1488-95. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Kim DH, O’Toole JE, Ogden AT, et al. Minimally invasive posterolateral thoracic corpectomy: cadaveric feasibility study and report of four clinical cases. Neurosurgery 2009;64:746-52; discussion 752-3. [Crossref] [PubMed]
- Pennington Z, Ahmed AK, Molina CA, et al. Minimally invasive versus conventional spine surgery for vertebral metastases: a systematic review of the evidence. Ann Transl Med 2018;6:103. [Crossref] [PubMed]
- Billinghurst J, Akbarnia B. Extreme lateral interbody fusion - XLIF. Curr Orthop Pract 2009;20:238-51. [Crossref]
- Shin DA, Kim KN, Shin HC, et al. The efficacy of microendoscopic discectomy in reducing iatrogenic muscle injury. J Neurosurg Spine 2008;8:39-43. [Crossref] [PubMed]
- Lau D, Chou D. Posterior thoracic corpectomy with cage reconstruction for metastatic spinal tumors: comparing the mini-open approach to the open approach. J Neurosurg Spine 2015;23:217-27. [Crossref] [PubMed]
- Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003;98:149-55. [PubMed]
- Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 2008;90:62-9. [Crossref] [PubMed]
- O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery: Clinical article. J Neurosurg Spine 2009;11:471-6. [Crossref] [PubMed]
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85. [Crossref] [PubMed]
- Smith WD, Dakwar E, Le TV, et al. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine 2010;35:S338-46. [Crossref] [PubMed]
- McAfee PC, Phillips FM, Andersson G, et al. Minimally Invasive Spine Surgery. Spine 2010;35:S271-3. [Crossref] [PubMed]
- Moussazadeh N, Rubin DG, McLaughlin L, et al. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J 2015;15:1609-17. [Crossref] [PubMed]
- Regan JJ, Yuan H, McAfee PC. Laparoscopic Fusion of the Lumbar Spine: Minimally Invasive Spine Surgery: A Prospective Multicenter Study Evaluating Open and Laparoscopic Lumbar Fusion. Spine 1999;24:402-11. [Crossref] [PubMed]
- Smith ZA, Yang I, Gorgulho A, et al. Emerging techniques in the minimally invasive treatment and management of thoracic spine tumors. J Neurooncol 2012;107:443-55. [Crossref] [PubMed]
- Deutsch H, Boco T, Lobel J. Minimally Invasive Transpedicular Vertebrectomy for Metastatic Disease to the Thoracic Spine. J Spinal Disord Tech 2008;21:101-5. [Crossref] [PubMed]
- Chou D, Lu DC. Mini-open transpedicular corpectomies with expandable cage reconstruction: Technical note. J Neurosurg Spine 2011;14:71-7. [Crossref] [PubMed]
- Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis--prospective study. Radiology 1990;176:279-82. [Crossref] [PubMed]
- Lu DC, Chou D, Mummaneni PV. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors: Clinical article. J Neurosurg Spine 2011;14:758-64. [Crossref] [PubMed]
- Musacchio M, Patel N, Bagan B, et al. Minimally invasive thoracolumbar costotransversectomy and corpectomy via a dual-tube technique: evaluation in a cadaver model. Surg Technol Int 2007;16:221-5. [PubMed]
- Zairi F, Vielliard MH, Bouras A, et al. Long-segment percutaneous screw fixation for thoraco-lumbar spine metastases: a single center’s experience. J Neurosurg Sci 2017;61:365-70. [PubMed]
- Tutton SM, Pflugmacher R, Davidian M, et al. KAST Study: The Kiva System As a Vertebral Augmentation Treatment-A Safety and Effectiveness Trial: A Randomized, Noninferiority Trial Comparing the Kiva System With Balloon Kyphoplasty in Treatment of Osteoporotic Vertebral Compression Fractures. Spine 2015;40:865-75. [Crossref] [PubMed]
- Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12:225-35. [Crossref] [PubMed]
- Korovessis P, Repantis T, Miller LE, et al. Initial clinical experience with a novel vertebral augmentation system for treatment of symptomatic vertebral compression fractures: A case series of 26 consecutive patients. BMC Musculoskelet Disord 2011;12:206. [Crossref] [PubMed]
- Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J. Clin. Oncol 2004;22:300-6. [Crossref] [PubMed]
- Thanos L, Mylona S, Galani P, et al. Radiofrequency ablation of osseous metastases for the palliation of pain. Skeletal Radiol 2008;37:189-94. [Crossref] [PubMed]
- Jang JS, Lee SH. Efficacy of percutaneous vertebroplasty combined with radiotherapy in osteolytic metastatic spinal tumors. J Neurosurg Spine 2005;2:243-8. [Crossref] [PubMed]
- Schneider F, Greineck F, Clausen S, et al. Development of a Novel Method for Intraoperative Radiotherapy During Kyphoplasty for Spinal Metastases (Kypho-IORT). Int J Radiat Oncol Biol Phys 2011;81:1114-9. [Crossref] [PubMed]
- Gao Z, Wu Z, Lin Y, et al. Percutaneous transforaminal endoscopic decompression in the treatment of spinal metastases: A case report. Medicine (Baltimore) 2019;98:e14819. [Crossref] [PubMed]
- Tsai SH, Wu HH, Cheng CY, et al. Full endoscopic interlaminar approach to nerve root decompression of sacral metastatic tumor. World Neurosurg 2018;112:57-63. [Crossref] [PubMed]
- McLain RF. Spinal cord decompression: an endoscopically assisted approach for metastatic tumors. Spinal Cord 2001;39:482-7. [Crossref] [PubMed]


