Micro-anatomical structures of the lumbar intervertebral foramen for full-endoscopic spine surgery: review of the literatures
Introduction
Percutaneous endoscopic lumbar discectomy (PELD) can be performed via the transforaminal (TF) or interlaminar approach. The typical TF approach is guided by Kambin’s triangle (also known as the safety triangle) (1); an endoscope is inserted through the intervertebral foramen to observe and treat dural ventral lesions in the spinal canal. Having knowledge and understanding of the intervertebral foramen and surrounding microanatomy is essential because these influences the surgical indications and results. Understanding the microanatomy of the Kambin’s triangle over the intervertebral foramen is also required when the intermuscular posterolateral (Wiltse) approach is followed and TF lumbar interbody fusion (TLIF) is performed. Previous studies will be reviewed and outlined in this manuscript.
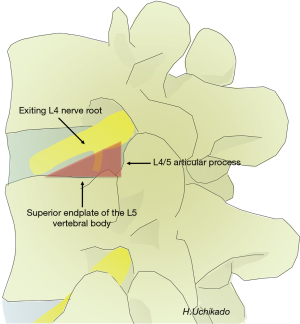
Lumbar intervertebral foramen and Kambin’s triangle
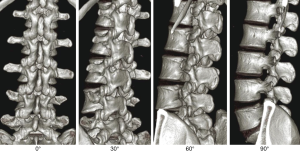
The shape of the lumbar intervertebral foramen differs depending on the direction from which it is viewed. Three-dimensional computed tomography (3D-CT) of the lumbar spine in healthy individuals shows that the intervertebral foramen cannot be identified from the dorsal side but gradually becomes visible when viewed laterally at a 30° angle and is maximized at a 90° lateral angle from the left. From the superior L1–2 intervertebral foramen to the inferior L4–5 level, progressive widening is observed. The L5–S1 intervertebral foramen cannot be identified if it is viewed from an angle of >60° laterally because of the iliac crest (Figure 1). The lumbar intervertebral foramen is oval with a height of 19.4 mm (range, 15.5–24.2 mm) and a width of 8.8 mm (range, 6.4–12.3 mm), with the maximum at the L5–S1 foramen (2). Kambin’s triangle is a 3D right triangle that is a key fluoroscopic indicator for punctures in discography and nerve root blocks (1). Kambin’s triangle in the two-dimensional plane is a triangle wherein the base is the inferior vertebral upper endplate, height is the foraminal side of the superior articular process, and oblique edge is the superior exiting nerve root (3) (Figure 2). Measurements of Kambin’s triangle in cadavers revealed that the area of the triangle becomes wider as one moves to the lower level of the lumbar spine (L4–5) (3). It is of great importance to have knowledge of the safe working zone with respect to TLIF and the TF approach for PELD while performing surgery (4,5). The relationship between the course of the nerve root and intervertebral discs and foramen is such that the angle of the nerve root bifurcation becomes steeper in the coronal section as one moves to the L5 nerve root (lower level) (6,7). In the sagittal section, the angle becomes more relaxed (ventral shift) as one moves to the L5 nerve root (lower level) (7). Based on the course of the exiting nerve root, Kambin’s triangle becomes narrower in L5/S1 than in L4–5 (5). Furthermore, the position of the ganglion shifts from within the intervertebral foramen into the spinal canal as one moves to the L5 nerve root (lower level). Therefore, pain is easily induced in herniated discs/prolapse of hernia (6,7). The intravertebral nerve root is maximal at L4 (mean, 3.9 mm) and minimal at L1 (mean, 3.3 mm) (1). Each nerve root shifts to the caudal side from the cranial side of the intervertebral foramen and runs more centrally as one moves to the lower lumbar level (8). Thus, the safest puncture site is the site closer to the right angle of Kambin’s triangle. Kambin’s triangle becomes narrow based on the reduction in the height of the intervertebral disc with the degeneration of intervertebral discs and joints associated with aging.
Ligament and vascular anatomy outside the lumbar intervertebral foramen
Inter-transverse ligaments (ITL) can be found in the group of muscles (from the inside, multifidus muscle, longissimus muscle, and iliocostalis muscle) on the posterodorsal side of the lumbar spine toward the intervertebral foramen. The lateral part of the facet joint is the entrance of the lateral part of the intervertebral foramen. There is a muscular branch called the dorsal branch between the ITL and the isthmus from the segmental arteries (9). The dorsal ramus (DR) runs along the periphery of the ganglion. It runs between the lower level mastoid and accessory processes to the facet joints inferior to one vertebra (10); for example, the DR from the periphery of the L3 ganglion passes between the mastoid and accessory processes of L4 to the L4–5 joint capsule. ITLs at the lumbosacral junction (L5–S1) are lumbosacral ligaments (11).
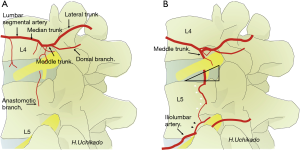
If the ITL is removed, there will be 2 layers of ligaments outside the intervertebral foramen. The first layer will consist of 2 strong corporotransverse ligaments (CTL), superior CTL passing from the superior border of the lower vertebral body to the superior transverse process and inferior CTL passing from the inferior border of the superior vertebral body to the inferior transverse process (Figure 3A).

The second layer will consist of the upper and lower ligaments and the extraforaminal ligament (EFL) anchoring the nerve root (Figure 3B). Removing the EFL has been shown to improve the mobility of nerve roots (L3–5, maximal at L5) (12,13). Caglar reported that CTLs were Y-ligaments. These are divided into 3 compartments in the form of an inverted Y. In the anterolateral compartment, there exists the anterior branch of the nerves and median trunk of the lumbar arteriovenous; in the posterolateral compartment, there exists the dorsal branch from the middle trunk; and in the middle compartment, there exists the posterior branch of the nerve and lateral trunk pass (11). The superficial layer of CTLs at the lumbosacral junction forms a site-specific lumbosacral hood (14).
There are 2 EFLs, and Kraan et al. reported that these ran in different directions in the T12–L1 and L2–5 nerve roots (13). The inferior EFL starts in L2–5 from the intervertebral disc (origin) and continues onto the ventrolateral side of the nerve root. This ligament anchors the extraforaminal nerve.
The arterial bifurcation is divided from the abdominal aorta to the medial, middle, and lateral trunk from each segmental artery of L1–4 (segmental or lumbar artery) (Figure 4A). From the median trunk, after branching of the lumbar plexus and muscular branches, the spinal and ganglionic branches bifurcate into the ventral side of the intervertebral foramen head. Dorsal and anastomotic branches separate from the middle trunk (15,16). Ultimately, the lateral trunk reaches the abdominal wall. With respect to the spinal branch, the Adamkiewicz artery that branches out from the lumbar arteries is not always rare and is found in T12–L1 (16%), L1–2 (21.4%), and L2–4 (6%). It is necessary to pay attention to the development of spinal cord infarction during surgery (17). There is no lumbar artery for the L5 spinal branch, which branches from the iliolumbar artery from the internal iliac artery or median sacral artery. In the segmental artery, there is a communicating branch that runs longitudinally from the ventral side of the intervertebral foramen to the ventral side of the transverse process. This communicating branch is seen at each level and found in approximately half of in the cadaver study (16). This communicating branch runs approximately 4 mm anterior to the transverse process. Because the L5 lumbar artery is absent, the supply is obtained from the iliolumbar artery from the internal iliac artery or medial sacral artery. In case the supply is from the medial sacral artery, there is no communicating branch because there is no branching to the L5-S1 intervertebral foramen. If communicating branches are noted between L4 and L5, they are the thickest (0.4–0.42 mm) (16). This communicating branch runs through Kambin’s triangle ventral to the L4–5 foramen (Figure 4B). Particularly, damage to these structures seems to be the cause of retroperitoneal hematoma (18). Therefore, in patients who are suspected to have communicating branches, confirming and obtaining knowledge of the course of blood vessels in preoperative examinations is important (19).
In PELD and retroperitoneal organs, it is necessary to pay attention to the kidneys and peritoneum in patients with L1–3 lesions. Particular attention must be paid to the pudendal femoral nerve in the lumbar plexus; however, this is less problematic because Kambin’s triangle is used as an indicator (20). Preoperative evaluations of the vascular anatomy surrounding the intervertebral foramen are extremely important to prevent complications. At the time of puncture, the right angle near the inferior superior articular process is more safety.
Ligament and vascular anatomy within the lumbar intervertebral foramen
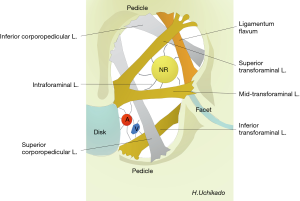
Akdemir et al. reported on the intraforaminal ligament (seventh foraminal ligaments) (21). The ligament is fixed around the nerve root in the intervertebral foramen. There are 3 other TF ligaments in the intervertebral foramen, the superior, middle, and inferior ligaments; 2 superior and inferior corporopedicular ligaments; and the ligamentum flavum (Figure 5). The anatomical proof of the nerve stretch test was reported by the presence of the intraforaminal ligament. The L5–S1 intraforaminal ligaments differs from the L1–5 levels, and Zhao et al. reported the presence of superior, inferior, anterior, and posterior radiating ligaments (22).
Of the arteries that pass through the intervertebral foramen, the spinal and ganglionic branches are critical (Figure 6). Results of study involving the use of 3D-CT angiography (3D-CTA) to confirm the presence of arteries in the intervertebral foramen have reported that arteries are also present within Kambin’s triangle (23). Therefore, because performing 3D-CTA in PELD is considered to be an important preoperative examination.
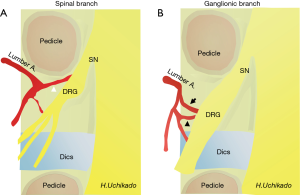
Variations in the course of the anterior and posterior nerve roots in the intervertebral foramen have been reported in detail by Kikuchi and Sato et al. (24-26). These variations have been suggested to also influence the clinical symptoms and efficacy of blocks or decompression. Cho et al. have reported on the relationships between the arterial course and dura mater around the dorsal root ganglion (DRG) and presented histological reports on the anterior and posterior roots (27). After dural penetration, the spinal and ganglion branches run along the anterior root, rather than the dorsal root; become the radicular artery; and run within the dura.
The nerve root is fixed by the ligament, and caution is required when utilizing a PELD cannula in the nonmobile foramen (Figure 7). Various reports on minimizing exiting nerve root injuries have been published (28,29). It is considered safe to perform foraminoplasty (30) and establish an approach or perform surgery under with the guidance of microscopy in patients with degeneration and intervertebral foraminal stenosis.
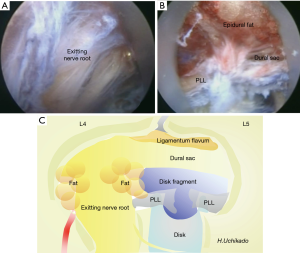
Spinal canal viewed through the intervertebral foramen
Spinal canal, which can be viewed through the intervertebral foramen, is the articular segment. This site comprises the intervertebral disc, ligamentum flavum, inferior superior articular process, and superior and inferior vertebral endplates. Degeneration of the articular segment is the cause of spinal canal and foraminal stenosis. The region surrounded by the vertebral arch (without the ligamentum flavum), vertebrae, and pedicle is the osseous segment; the epidural space on the dural ventral side of the vertebral posterior surface becomes wider at L4 and L5 than at L3 (31). The articular segment contains the ligamentum flavum (dorsal to the spinal canal) and posterior longitudinal ligament (ventral). The dorsal ligamentum flavum adheres to the superior margin of the caudal lamina from the middle and lower one-third of the ventral arch of the temporal lamina. The lateral side reaches the ventral side of the facet joint at the intervertebral foramen and migrates to the joint capsule and periosteum. Conversely, the posterior longitudinal ligament of the ventral side runs longitudinally in the center of the dorsal surface of the vertebral body. In the articular segment at the level of the intervertebral disc, the superficial layer shifts outward, becoming an epidural membrane and forming an epiradicular sheath. Abundant adipose tissue exists in the nerve root axilla, where the epidural venous plexus develops simultaneously (Figure 8A). Venous anatomy is more important than arteries in the spinal canal. When following the TF approach for PELD, internal vertebral venous layers that run longitudinally in front of the posterior longitudinal ligament on the ventral side of the spinal canal are noted (Figure 8B). Intravenous disruption has been reported in patients with herniated discs (32).
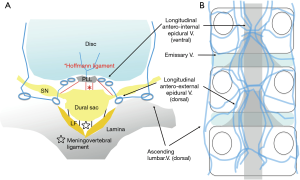
The dural sac is surrounded by a fibrous connective tissue, the epidural membrane. This thin membranous tissue contains abundant adipose tissue and is loosely connected to the right and left ligamentum flavum and posterior longitudinal ligament at the dorsal and ventral midline, that is, the dural sac is fixed by epidural ligaments and meningovertebral ligaments, which are located ventrally and dorsally to the spinal canal (Figure 8A). As initial findings during L5 laminectomy, Solaroglu et al. reported the presence of 1–2 ligaments, attention to terminal attachment (ATA) ligaments (33), connected from the dorsal surface of the dural sac to the medial cranial side of the ligamentum flavum. Damage to the ATA causes dural injuries and subsequent postoperative cerebrospinal fluid (CSF) leakage. Using cadavers, Shi et al. reported on endoscopic dissection at the lumbosacral level (34). Along the caudal side, the presence and number of ligament (0–2 or 3; width, approximately 0.6 mm; length, approximately 16 mm; thickness, 0.15 mm) increase, and the ligaments become the thickest and strongest at the sacral level. Because continuity with epidural fat vessels is also observed, the injury may cause intraoperative and postoperative epidural hemorrhage (18). On the ventral side of the dura, the so-called Hoffmann ligaments (35), which are attached slightly cranial to the nerve root sac from the superficial layer of the posterior longitudinal ligament, start from the periosteum and epidural membrane inside the pedicle (Figure 8A). These fibers are involved in forming the lateral root epiradicular sheath (29). Mobility of the nerve root changes significantly after resection of these Hoffmann ligaments. Injury to the ventral dura mater of the Hoffmann ligaments can result in CSF leakage, and consequently, prolapse of the herniated nerve roots (36) and herniated disc into the dura mater (37) can occur; therefore, such injuries should be handled with extra care.
With recent improvements in preoperative imaging diagnosis techniques, presence of transitional vertebrae and abnormalities in the course of nerves in the lumbosacral vertebrae can be predicted preoperatively. A TF approach should be avoided if anomalies in the course of nerves are suspected. During surgery, an unexpected anomaly in the course of nerves may be encountered. Electrophysiological functional anatomy can be expected, and if encountered, identifying the type of anomalies in the course of nerve roots (38,39) and performing endoscopic imaging are important. Endoscopic images during surgery and schemas of anatomical structures within the spinal canal visible through the intervertebral foramen are presented in Figure 7A,B,C, respectively.
Conclusions
Lastly, having knowledge of the anatomy around the lumbar intervertebral foramen is essential while following the TF approach for PELD techniques. Moreover, performing preoperative imaging is important for the success of the procedure, which leads to the prevention of complications and better diagnostic and therapeutic outcomes.
Acknowledgments
The authors thank Crimson Interactive Pvt. Ltd. (Ulatus)—www.ulatus.jp for their assistance in manuscript translation and editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.10.07). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs: report of interim results. Clin Orthop Relat Res 1986.37-43. [PubMed]
- Torun F, Dolgun H, Tuna G, et al. Morphometric analysis of the roots and neural foramina of the lumbar foramina. Surg Neurol 2006;66:148-51. [Crossref] [PubMed]
- Hoshide R, Feldman E, Taylor W. Cadaveric analysis of the Kambin’s triangle. Cureus 2016;8:e475. [PubMed]
- Hardenbrook M, Lombardo S, Wilson MC, et al. The anatomic rationale for transforaminal interbody fusion: a cadaveic analysis. Neurosurg Focus 2016;40:E12. [Crossref] [PubMed]
- Lertudomphonwanit T, Kaorochana G, Kraiwattanapon C, et al. Anatomic considerations of intervertebral disk perspective in lumbar posterolateral approach via Kambin’s triangle cadaveric study. Asian Spine J 2016;10:821-7. [Crossref] [PubMed]
- Suh SW, Shingade VU, Lee SH, et al. Origin of lumbar spinal roots and their relationship to intervertebral disk. a cadaver and radiological study. J Bone Joint Surg 2005;87:512-22.
- Wu YS, Lin Y, Zhang XL, et al. The projection of nerve roots on the posterior aspect of spine from T11 to L5. A cadaver and radiological study. Spine (Phila Pa 1976) 2012;37:E1232-7. [Crossref] [PubMed]
- Arslan M, Comert A, Acar H, et al. Nerve root to lumbar disc relationships at the intervertebral foramen from a surgical viewpoint: An anatomical study. Clin Anat 2012;25:218-23. [Crossref] [PubMed]
- Viswanathan R, Swami NK, Tobler WD, et al. Extraforaminal lumbar disc herniations: microsurgical anatomy and surgical approach. J Neurosurg 2002;96:206-11. [PubMed]
- Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg 1979;51:172-7. [Crossref] [PubMed]
- Caglar Y YS, Dolgun H, Ugur HC, et al. A ligament in the lumbar foramina: inverted Y ligament: an anatomic report. Spine (Phila Pa 1976) 2004;29:1504-7. [Crossref] [PubMed]
- Grimes PF, Massive JB, Grain SR. Anatomic and biomechanical analysis of the lower lumbar foraminal ligaments. Spine (Phila Pa 1976) 2000;25:2009-14. [Crossref] [PubMed]
- Kraan GA, Delwel EJ, Hooglant PV, et al. Extraforaminal ligament attachments of human lumbar nerves. Spine (Phila Pa 1976) 2005;30:601-5. [Crossref] [PubMed]
- Amonoo-Kuofi HS, El-Badawi MG, Gatani JA, et al. Ligaments associated with lumbar intervertebral foramina. 2. The fifth lumbar level. J Anat 1988;159:1-10. [PubMed]
- Caglar S, Dolgun H, Ugur HC, et al. Extraforaminal lumbar arterial anatomy. Surg Neurol 2004;61:29-33. [Crossref] [PubMed]
- Arslan M, Comert A, Acar HI, et al. Surgical view of the lumbar arteries and their branches: an anatomical study. Neurosurgery 2011;68:16-22; discussion 22. [PubMed]
- Illuminati G, Koskas F, Bertagni A, et al. Variations in the origin of the artery of Adamkiewicz. Riv Eur Sci Med Farmacol 1996;18:61-6. [PubMed]
- Ahn Y, Kim JU, Lee BH, et al. Postoperative retroperitoneal hematoma following transforaminal endoscopic lumbar discectomy. Clinical article. J Neurosurg Spine 2009;10:595-602. [Crossref] [PubMed]
- Sakai T, Tezuka F, Wada K, et al. Risk Management for Avoidance of Major Vascular Injury due to Lateral Transpsoas Approach. Spine (Phila Pa 1976) 2016;41:450-3. [Crossref] [PubMed]
- Moro T, Kikuchi S, Konno S, et al. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976) 2003;28:423-8; discussion 427-8. [Crossref] [PubMed]
- Akdemir G. Thoracic and lumbar intraforaminal ligaments. Laboratory investigation. J Neurosurg Spine 2010;13:351-5. [Crossref] [PubMed]
- Zhao Q, Zhong E, Shi B, et al. The morphology and clinical significance of the intraforaminal ligaments at the L5-S1 levels. Spine J 2016;16:1001-6. [Crossref] [PubMed]
- Simon JI, McAuliffe M, Smoker D. Location of radicular spinal arteries in the lumbar spine from analysis of CT angiogram of the abdomen and pelvis. Pain Medicine 2016;17:46-51. [PubMed]
- Kikuchi S, Hasue M, Nishiyama K, et al. Anatomic and clinical studies of radicular symptoms. Spine (Phila Pa 1976) 1984;9:23-30. [Crossref] [PubMed]
- Kikuchi S, Sato K, Konno S, et al. Anatomic and radiographic study of dorsal root ganglia. Spine (Phila Pa 1976) 1994;19:6-11. [Crossref] [PubMed]
- Sato K, Kikuchi S. An anatomic study of foraminal nerve root lesions in the lumbar spine. Spine (Phila Pa 1976) 1993;18:2246-51. [Crossref] [PubMed]
- Cho KH, Jin ZW, Abe H, et al. Neural-Dural Transition at the Thoracic and lumbar nerve roots: A histological study of human late-stage fetuses. Research Article. Biomed Res Int 2016;2016:8163519.
- Cho JY, Lee SH, Lee HY. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: floating retraction technique. Minim Invasive Neurosurg 2011;54:214-8. [Crossref] [PubMed]
- Choi KC, Kim JS, Park CC. Percutaneous endoscopic Lumbar Discectomy as an alternative to open lumbar microdiscectomy for large lumbar disc herniation. Pain Physician 2016;19:E291-300. [PubMed]
- Henmi T, Terai T, Hibino N, et al. Percutaneous endoscopic lumbar discectomy utilizing ventral epiduroscopic observation technique and foraminoplasty for transligamentous extruded nucleus purposes: technical note. J Neurosurg Spine 2016;24:275-80. [Crossref] [PubMed]
- Teske W, Kramer J, Lichtinger T, et al. A morphometric cadaver study of the anterior lumbar epidural space. Eur Spine J 2012;21:1479-82. [Crossref] [PubMed]
- Roland J, Treil J, Larde D, et al. Lumbar phlebography in the diagnosis of disc herniations. J Neurosurg 1978;49:544-50. [Crossref] [PubMed]
- Solaroglu I, Okutani O, Beskonakli E. The ATA and its surgical importance: a newly described ligament lying between the dural sac and the ligamentum flavum at the L5 level. Spine (Phila Pa 1976) 2011;36:1268-72. [Crossref] [PubMed]
- Shi B, Li X, Li H, et al. The morphology and clinical significance of the dorsal meningovertebra ligaments in the lumbosacral epidural space. Spine (Phila Pa 1976) 2012;37:E1093-8. [Crossref] [PubMed]
- Tardieu GG, Fisahn C, Loukas M, et al. The Epidural Ligaments (of Hofmann): A Comprehensive Review of the Literature. Cureus 2016;8:e779. [PubMed]
- Ahn Y, Lee HY, Lee SH, et al. Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J 2011;20:58-64. [Crossref] [PubMed]
- Tamaki Y, Sakai T, Miyagi R, et al. Intradural lumbar disc herniation after percutaneous endoscopic lumbar discectomy: case report. J Neurosurg Spine 2015;23:336-9. [Crossref] [PubMed]
- Neidre A, MacNab I. Anomalies of the lumbosacral nerve roots. Review of 16 cases and classification. Spine (Phila Pa 1976) 1983;8:294-9. [Crossref] [PubMed]
- McCulloch JA, Waddell G. Variation of the lumbosacral myotome bony segmental anomalies. J Bone Joint Surg Br 1980;62-B:475-80. [Crossref] [PubMed]