
A less invasive treatment by a full-endoscopic spine surgery for adjacent segment disease after lumbar interbody fusion
Introduction
Lumbar interbody fusion (LIF) is an established strategy for the treatment of lumbar degenerative diseases such as lumbar canal stenosis associated with a spondylolisthesis (1). Despite its excellent efficacy, adjacent segment disease (ASD) still occurs. In previous reports, the incidence rate of symptomatic ADS was reported to be 4.9–15.3% after a minimum 2-year follow-up (2,3). ASD is usually considered a relatively long-term complication, but early-onset ASD within 1 year after LIF was also reported to be 0.8% (8 of 1,000 cases) (4,5). Although several pathogenetic factors for ASD have already been identified (6), complete prevention of ASD is impossible because of their degenerative nature.
On the other hand, full-endoscopic spine surgery (FESS) has recently been developed and is one of the most sophisticated operative procedures for the treatment of lumbar disc herniation (LDH) (7). FESS can be applied not only for the treatment of LDH but also for the treatment of foraminal stenosis together with a high-speed drill (8). We have therefore applied FESS for the treatment of ASD after LIF. In this retrospective analysis, we summarize our experience, identify the technical hurdles, and present the operative outcomes for the treatment of ASD.
Methods
We analyzed consecutive patients with ASD after LIF underwent FESS between September 2015 and March 2019 with a 7-mm diameter spinal full-endoscopic system (Richard Wolf GmbH, Knittlingen, Germany). Exclusion criteria were as follows: (I) we could not fully complete the preoperative and postoperative evaluations. (II) we retrospectively confirmed the presence of an adjacent LDH before LIF at other hospital.
For all patients, FESS was conducted at only one vertebral level by a single surgeon (H Koga). Neurological examination, electrophysiological examination, preoperative magnetic resonance imaging (MRI), and computed tomography (CT) were used to diagnose ASD after LIF.
Patients were followed for an average of 13.1 months (range, 2–45 months) postoperatively. Preoperative and postoperative neurological statuses were evaluated using the modified Japanese Orthopaedic Association (mJOA) score for lumbar diseases (9,10). The recovery rate was determined as follows: recovery rate = (postoperative mJOA − preoperative mJOA)/[23 (full score) − preoperative mJOA score] ×100. Corresponding leg pain was also evaluated using the Numerical Rating Scale (NRS) score. Statistical analysis was performed using Students’ t-test. P values less than 0.05 were considered statistically significant.
Surgical technique
The patients were carefully log-rolled into a prone position. The surgery was performed under general anesthesia and simultaneous motor evoked potential monitoring. During the operation, a fluoroscope was placed across the center of the operating table to ensure appropriate positioning. Eight-millimeter ipsilateral skin and fascial incisions were made on the corresponding vertebral level. The basic operative procedures for LDH [full-endoscopic discectomy (FED)-transforaminal (TFA), -interlaminar (ILA), and -posterolateral approaches (PLA)] (11) and for foraminal stenosis [full-endoscopic laminectomy-translaminar approach (FEL-TLA)] were described previously (8). In addition to these basic FESS procedures, foraminoplasty was also described in our previous study (7). In all operations, former established fixation instruments (screws and rods) were not disturbed during the FESS procedures.
Results
Thirteen patients were registered for this study, of which 10 were men and 3 were women. The mean patient age was 64.8 years (range, 45–83 years). We performed FESS on 16 patients with ASD after LIF in this term. Of these 16 patients, we excluded 2 who did not fully complete the preoperative and postoperative evaluations. We also excluded 1 patient who underwent LIF at other university hospitals because the patient’s symptoms did not change after LIF, and retrospective analysis of the patient’s preoperative MRI revealed the presence of an adjacent LDH before LIF. Previous LIF was performed on 6 patients in our hospital and on 7 patients in other hospitals. ASD symptoms appeared in various periods after LIF (average, 63.3 months; range, 1–166 months). Except for 1 case (case 8, bilateral radiculopathy and muscle weakness), 12 patients had unilateral radiculopathy that was resistant to medical treatment, epidural steroid injection, and/or nerve block. The average period between FESS and symptom appearance was 8.2 months (range, 1–26 months) (Table 1).
Full table
Detailed disease states are described in Table 1; 9 cases of LDH and 4 cases of foraminal stenosis were observed. Even in cases of LDH, foraminal stenosis was also present in some cases. Therefore, additional foraminoplasty was performed in 6 out of 9 cases of LDH. The mean operative time was 52.7 min (range, 36–68 min), and the mean blood loss was negligible in all patients. Although we observed one operative complication [surgical site infection (SSI)] in this case series, SSI was cured by re-operation (irrigation) and antibiotics (Table 2).
Full table
The mean postoperative hospital stay was 1.5 days (range, 1–5 days); it was particularly long for case 4 because of symptoms of the common cold and abdominal pain (Table 2). During the follow-up period (range, 2–45 months; average, 13.1 months), leg pain stemming from ASD after LIF improved in 12 of 13 cases. The mean NRS scores statistically improved from 7.6±2.6 to 3.1±2.7 (Table 2). The mean mJOA score also improved from 10.5±4.2 to 16.1±4.9; the recovery rate of this score was 32.8%±63.4%. However, the extent was diverse and mJOA score was worse in 3 cases (cases 3, 6, and 9).
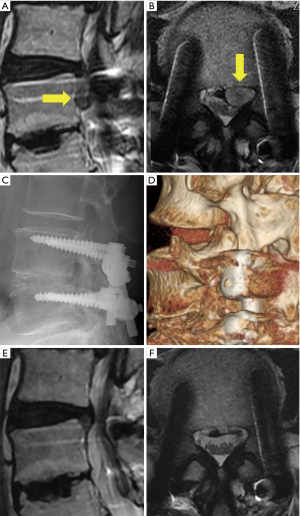
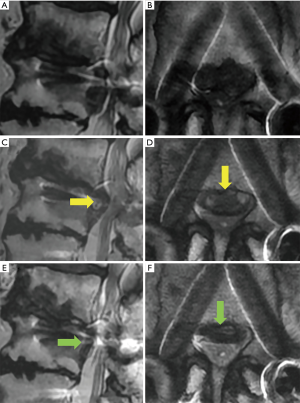
A representative case (case 12) is shown in Figure 1. This 50-year-old man presented with paresthesia of the left leg (L4 dermatomes) that started 10 years after LIF [L4/5 posterolateral interbody fusion (PLIF)] at another hospital. Neurological examination revealed a negative straight leg raise on both sides but severe muscle weakness (quadriceps muscle: 5/2). Lumbar MRI revealed left L3/4 LDH, which largely sequestrated to the caudal site with compression not only of the nerve root but also of the cauda equina (Figure 1A,B). A plain roentgenogram showed that the foraminal length and width were preserved (Figure 1C). A CT scan also demonstrated less osteoarthropathic changes of the corresponding left L3/4 facet joint (Figure 1D). Immediately after the operation, the patient’s leg paresthesia improved. Postoperative MRI revealed the disappearance of LDH and enlargement of the spinal canal, respectively (Figure 1E,F).
Discussion
ASD after LIF is a pathological condition in which degenerative changes occur at the vertebral disc and facet joint adjacent to the fused vertebrae. Subsequently, symptomatic LDH and/or foraminal stenosis accrue. For the treatment of this pathological condition, partial destruction of the facet joint is frequently required. So far, extension of the vertebrae fixation toward the adjacent vertebral level has been considered for the treatment in general. Additional fixation has a potential of further ASD (12) and complications based on instrumentation such as screw loosening and broken rod (13). To avoid these problems, we applied FESS to ASD after LIF.
In this article, we summarized 13 cases of ASD after LIF treated by FESS via different types of approaches (TFA, ILA, posterolateral, and translaminar). The mean preoperative and postoperative NRS scores were 7.6 and 3.1, respectively. The mean pre- and postoperative mJOA scores were 10.5 and 16.1, respectively, and the mean recovery rate was 32.8%. Compared with our previous FESS outcomes (7,8,14), the outcome in this study was inferior. The following are the reasons that are thought to have given rise to the inferior outcomes: (I) elderly patients, (II) longer disease duration (from ASD to FESS), and (III) more complicated pathological status.
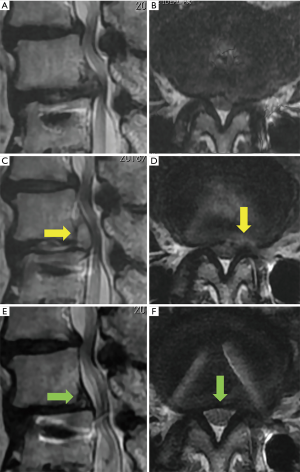
Moreover, 3 out of 13 patients required additional surgical treatment. The re-operation rate was higher than that of previous our FESS experiences. One patient (case 9, Figure 2) received subsequent FESS for the treatment of postoperative complication (SSI). In this case, the leg pain improved immediately after the first FESS and the patient was discharged; however, 20 days after the operation, the patient visited our outpatient clinic complaining of left leg pain and back pain. Laboratory data revealed the occurrence of SSI (Figure 2C,D). We therefore removed the purulent matter and washed the cavity using the same FESS procedure (Figure 2E,F). The patient recovered from the infection, but still complained of back pain due to the regional instability or destruction of the terminal plate. In our previous experiences with FESS (more than 1,000 cases), we only experienced 2 cases of infections (including this case). Another case of infection involved a relatively early stage of introduction of FESS in our hospital. The operative time was 101 min, which was longer than that of case 9 (44 min). Postoperative infections may occur at a higher rate in FESS for ASD after LIF than for ordinary FESS.
Another patient (case 8, Figure 3) received subsequent FESS for the treatment of insufficient decompression. This case presented with a large central type of LDH (Figure 3A,B) and could not walk because of severe bilateral leg pain. After the first FESS from the left side (Figure 3C,D), the patient’s symptoms partially improved but he still complained of severe right leg pain. After contralateral FESS 6 months after the first FESS (Figure 3E,F), the patient had considerably less leg pain and could walk with a cane.
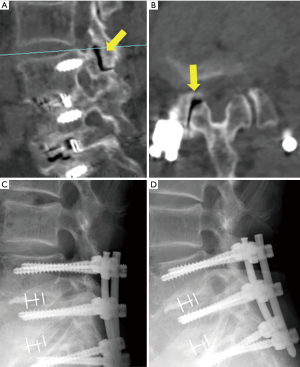
The remaining case (case 3) underwent a second FESS 5 months after the first FESS against recurrence LDH. To clarify the factor for early recurrence, we retrospectively analyzed the radiographical data. In this case, we found intraarticular vacuum phenomenon at the adjacent facet joint (Figure 4A,B, arrows) and sharp angle of the joint on axial CT image (Figure 4B, right angle =77.4°). We could not observe severe instability [gross motion (>3 mm) on flexion-extension lumbar lateral X-roentgenogram] (Figure 4C,D). As this was the only case of recurrence, we could not conclude that intraarticular vacuum phenomenon and sharp angle of the adjacent facet joint (Figure 4A,B, arrows) were the factors for failure of FESS. Vacuum facet phenomena and facet angle >50° were reported the factor of instability in degenerative spondylolisthesis and delayed instability following decompression without fusion, respectively (15,16). It is necessary to accumulate the data regarding condition of the adjacent facet joint; nevertheless, vacuum facet phenomena and facet angle may be predictive factors to select FESS or extension of the vertebrae fixation.
Although the re-operation rate (23%) was higher than that of ordinary FESS, all cases recovered after a second similar attempt. McGrath et al. also reported similar findings in their early experience with endoscopic treatment of ASD after LIF (17). Although 2 out of 7 patients required further revision surgeries, one patient was cured by a second FESS.
Very recently, similar studies to ours have been published from several investigators (17-24). All studies showed excellent outcomes of FESS for the treatment of ASD after LIF. Among these studies, Ba et al. compared the postoperative outcome between 33 cases of FESS and 31 cases of LIF, and showed similar improvement rates (82.75% and 86.28%, respectively) (23). Sun et al. also compared the postoperative outcome between 11 cases of FESS and 13 cases of LIF and showed that FESS was excellent in terms of operative time, bleeding volume, recovery period, and financial support (24). Furthermore, Gu et al. especially emphasized the usefulness of FESS for elderly patients (22). However, Telfeian et al. observed a relatively high 2-year failure rate (33%, 3 of 9 cases) and concluded that the benefit of this technique may be temporary (21).
We could not obtain fully satisfactory results from this case series because of some difficulties during the FESS treatment of ASD after LIF. But extension of the fusion to the adjacent vertebrae or sacrum is impossible to reverse. We therefore have to carefully select an appropriate operative strategy for ASD after LIF. Furthermore, from this study, we could not obtain insight into the factors that promise excellent therapeutic effects of FESS for the treatment of ASD after LIF. More patients and a longer follow-up period in a future study can clarify such factors. For this purpose, we are now accumulating data regarding the anatomical structures and radiographic findings to identify predictive factors that promise successful FESS treatment outcomes.
Limitations of the study include the small number of samples, the short follow-up period, the lack of comparative data with a group of patients in which they performed an additional LIF for dealing with ASD, and the lack of other evaluation methods for clinical outcomes such as Short Form 36 questionnaire (SF-36) and Oswestry disability index (ODI).
Conclusions
The preliminary results obtained during our short follow-up period showed that FESS was feasible for the treatment of ASD after LIF. However, elderly patients, longer disease duration, and more complicated pathological statuses resulted in inferior outcomes compared with those of ordinary FESS.
Acknowledgments
We thank all of the operating room staff for their technical assistance and the medical records clerks who helped to collect patient data. We also thank all radiological department staff for recording CT and MRI data.
Funding: This work was partly supported by a grant from the Iwai Medical Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.08.04). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. HK served as the unpaid Guest Editor of the series and serves as an unpaid editorial member of Journal of Spine Surgery from October 2018 to October 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by our ethical committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Resnick DK, Watters WC 3rd, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 2014;21:54-61. [Crossref] [PubMed]
- Kaito T, Hosono N, Mukai Y, et al. Induction of early degeneration of the adjacent segment after posterior lumbar interbody fusion by excessive distraction of lumbar disc space. J Neurosurg Spine 2010;12:671-9. [Crossref] [PubMed]
- Makino T, Honda H, Fujiwara H, et al. Low incidence of adjacent segment disease after posterior lumbar interbody fusion with minimum disc distraction. Medicine 2018;97:e9631. [Crossref] [PubMed]
- Okuda S, Yamashita T, Matsumoto T, et al. Adjacent Segment Disease After Posterior Lumbar Interbody Fusion: A Case Series of 1000 Patients. Global Spine J 2018;8:722-7. [Crossref] [PubMed]
- Matsuoka Y, Endo K, Suzuki H, et al. Postoperative Radiographic Early-Onset Adjacent Segment Degeneration after Single-Level L4-L5 Posterior Lumbar Interbody Fusion in Patients without Preoperative Severe Sagittal Spinal Imbalance. Asian Spine J 2018;12:743-8. [Crossref] [PubMed]
- Zhang C, Berven SH, Fortin M, et al. Adjacent Segment Degeneration Versus Disease After Lumbar Spine Fusion for Degenerative Pathology: A Systematic Review With Meta-Analysis of the Literature. Clin Spine Surg 2016;29:21-9. [Crossref] [PubMed]
- Fujita M, Kawano H, Kitagawa T, et al. Preoperative Design for the Posterolateral Approach in Full-Endoscopic Spine Surgery for the Treatment of L5/S1 Lumbar Disc Herniation. Neurospine 2019;16:105-12. [Crossref] [PubMed]
- Ishibashi K, Oshima Y, Inoue H, et al. A less invasive surgery using a full-endoscopic system for L5 nerve root compression caused by lumbar foraminal stenosis. J Spine Surg 2018;4:594-601. [Crossref] [PubMed]
- Azimi P, Mohammadi HR, Montazeri A. An outcome measure of functionality and pain in patients with lumbar disc herniation: a validation study of the Japanese Orthopedic Association (JOA) score. J Orthop Sci 2012;17:341-5. [Crossref] [PubMed]
- Takahashi T, Hanakita J, Minami M, et al. Surgical outcome and postoperative work status of lumbar discogenic pain following transforaminal interbody fusion. Neurol Med Chir (Tokyo) 2011;51:101-7. [Crossref] [PubMed]
- Yokosuka J, Oshima Y, Kaneko T, et al. Advantages and disadvantages of posterolateral approach for percutaneous endoscopic lumbar discectomy. J Spine Surg 2016;2:158-66. [Crossref] [PubMed]
- Louie PK, Varthi AG, Narain AS, et al. Stand-alone lateral lumbar interbody fusion for the treatment of symptomatic adjacent segment degeneration following previous lumbar fusion. Spine J 2018;18:2025-32. [Crossref] [PubMed]
- Ghobrial GM, Williams KA, Arnold P, et al. Iatrogenic neurologic deficit after lumbar spine surgery: A review. Clin Neurol Neurosurg 2015;139:76-80. [Crossref] [PubMed]
- Koga H, Inanami H. Minimal laminectomy using the interlaminar approach for percutaneous endoscopic lumbar discectomy. Mini-invasive Surg 2017;1:56-62. [Crossref]
- Sun ZM, Jiang C, Xu JJ, et al. Vacuum Facet Phenomenon in Computed Tomography Imaging: A Sign of Instability in Degenerative Spondylolisthesis? World Neurosurg 2019;129:e393-e400. [Crossref] [PubMed]
- Blumenthal C, Curran J, Benzel EC, et al. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J Neurosurg Spine 2013;18:340-6. [Crossref] [PubMed]
- McGrath LB Jr, Madhavan K, Chieng LO, et al. Early experience with endoscopic revision of lumbar spinal fusions. Neurosurg Focus 2016;40:E10. [Crossref] [PubMed]
- Wu JJ, Chen HZ, Zheng C. Transforaminal Percutaneous Endoscopic Discectomy and Foraminoplasty after Lumbar Spinal Fusion Surgery. Pain Physician 2017;20:E647-51. [PubMed]
- Telfeian AE. Endoscopic foraminotomy for recurrent lumbar radiculopathy after TLIF: Technical report. Surg Neurol Int 2015;6:62. [Crossref] [PubMed]
- Telfeian AE, Jasper GP, Francisco GM. Transforaminal endoscopic treatment of lumbar radiculopathy after instrumented lumbar spine fusion. Pain Physician 2015;18:179-84. [PubMed]
- Telfeian AE. Transforaminal Endoscopic Surgery for Adjacent Segment Disease After Lumbar Fusion. World Neurosurg 2017;97:231-5. [Crossref] [PubMed]
- Gu G, Wang C, Gu X, et al. Percutaneous Transforaminal Endoscopic Discectomy for Adjacent Segment Disease After Lumbar Fusion in Elderly Patients Over 65 Years Old. World Neurosurg 2018;112:e830-6. [Crossref] [PubMed]
- Ba Z, Pan F, Liu Z, et al. Percutaneous endoscopical transforaminal approach versus PLF to treat the single-level adjacent segment disease after PLF/PLIF: 1-2 years follow-up. Int J Surg 2017;42:22-6. [Crossref] [PubMed]
- Sun Y, Zhang W, Qie S, et al. Comprehensive comparing percutaneous endoscopic lumbar discectomy with posterior lumbar internal fixation for treatment of adjacent segment lumbar disc prolapse with stable retrolisthesis: A retrospective case-control study. Medicine (Baltimore) 2017;96:e7471. [Crossref] [PubMed]


