
The utilization of minimally invasive surgery techniques for the treatment of spinal deformity
Introduction
The treatment adult spinal deformity (ASD) continues to evolve. Surgical algorithms may include open, minimally invasive spine surgery (MISS) or hybrid approaches to decompress neural elements and restore global balance. When compared to open techniques, MIS approaches to ASD correction affords less blood loss, shorter hospital stays, and reduced morbidity (1). As the utility of MISS techniques and outcomes for scoliosis correction are better understood, the workflow of staged procedures will continue to evolve and improve.
Correction of global alignment and restoration of radiographic pelvic parameters has been correlated with improved outcomes and improved health-related quality-of-life (HRQOL) (2,3). Furthermore, open techniques carry a relatively high percentage of complications and prolonged hospital stay (2,4-6). MISS techniques with mini-open pedicle subtraction osteotomy (PSO) and anterior column realignment (ACR) with hyperlordotic interbodies allow for substantial changes in segmental lordosis and global sagittal balance (7-10).
The goals of surgical intervention for ASD are decompression to alleviate back and radicular pain with durable restoration of sagittal and coronal balance (11). The aim of this article is to review new and evolving MISS techniques, their applications to ASD correction, and the ability to achieve the aforementioned goals of surgical intervention.
Indications
ASD is an increasingly more recognized condition with a prevalence ranging from 8.3% as reported by Carter et al. in 1987 to a more contemporary incidence of 68% by Schwab et al. in 2005 (12,13). Numerous classifications systems exist for ASD and qualify it as a Cobb angle of greater than or equal to 10 degrees (14-17). The SRS-Schwab classification is a wholesome classification system taking into account multiple curve types, sagittal balance and association to pelvic parameters (17). Mummaneni et al. described the minimally invasive spinal deformity surgery (MISDEF) algorithm that can be used to guide decision making based on severity of deformity and spinopelvic parameters (18).
The most common presenting symptoms of ASD include disabling axial back pain, neurogenic claudication and/or lower extremity radicular complaints. The genesis of back pain in ASD is the progressive and asymmetric disc degeneration and facet arthropathy that may be precipitated by osteopenia/osteoporosis and/or compression fractures that cause asymmetric loading of the spine (19). Findings that relate to central stenosis can include spondylolisthesis and/or lateral listhesis. The concavity side can cause foraminal stenosis contributing to radicular complaints whereas the convexity can lead to nerve stretch and irritation. Correlating the patient’s symptoms to radiographic findings is key.
Risk factors associated with curve progression include a Cobb angle greater than 30 degrees, lateral listhesis of greater than 6mm, asymmetric disc space above or below the apical vertebra, and a deep-seated L5 vertebra relative to the intercrestal line (20,21). Indications for surgical intervention include new or progressive neurologic deficit, radicular pain and/or back pain that has been recalcitrant to conservative measures. Failure of conservative measures with multimodal pain management and physical therapy must be exhausted prior to surgical consideration.
Surgical planning
Minimally invasive surgical correction of ASD can minimize associated morbidity compared hybrid-techniques, however it does not obviate the accompanying risks (22). MISS techniques can be offered to patients via foraminotomy or laminectomy (23). Although there is a paucity of data regarding focal decompression in the setting of ASD, it may serve as an appealing, less invasive alternative for patients with more leg than back pain who are reluctant to undergo extensive thoracolumbar correction for ASD. Consideration of focal decompression alone can be considered in elderly patients with or without high-risk comorbid conditions and primary complaints of radicular pain. Patient counseling should include possible progression of scoliosis, deformity, or stenosis.
Short segment fusion has been offered as an alternative to long segment fixation and correction of ASD (24,25). In a review by Phan et al., long segment fusions (≥3 levels) were associated with, but not significant for a reduction in coronal Cobb angle and an increase in lumbar lordosis (25). Furthermore, there were no significant differences in peri-operative morbidity when comparing short and long constructs, but short constructs may shorten time and costs (25). Again, patients should be counseled on the possibility of progression of scoliosis and need for future surgery.
Long construct instrumentation and as needed decompression are the definitive surgical option for ASD and will be the focus of all subsequent discussion. Surgical planning should include correction of lumbar lordosis, sagittal and coronal balance while calculating interbody size/lordosis, and instrumented levels that generally extend from the lower thoracic levels to the pelvis. Minimally invasive techniques include anterior lumbar interbody fusion (ALIF), transforaminal lateral interbody fusion (TLIF), LLIF, ACR, mini-open PSO, and percutaneous pedicle screw placement.
As a matter of preference, the senior author prefers the transpsoas LLIF as access for interbody placement levels including the thoracolumbar junction down to the L4-5 level. The lateral transpsoas approach holds value in that it does not disrupt the facets nor the posterior or anterior ligamentous structures. Advantages include the ability to execute this approach without the need for an access surgeon, placement of large interbody grafts that allow for restoration of disc height and lordosis. LLIF interbodies bear a large footprint for fusion as well as central and foraminal indirect decompression of the neural elements (26-31). Contraindications to LLIF include the L5/S1 disc space, high grade spondylolisthesis, previous retroperitoneal surgery that may have caused scarring, vascular anomalies overlying the lateral/anterolateral vertebral body, significant osteophyte and/or ossification of the disc space.
Transforaminal lumbar interbody fusion is entertained when anatomic restrictions to the disc space inhibited by anatomic restrictions. Advantages to minimally invasive TLIF techniques include minimal to no nerve root retraction, ability to perform facetectomies, direct decompression, use of expandable cages, and the ability to perform interbody fusion in the same prone position as pedicle screw placement (32). As a matter of preference when considering TLIF for MISS correction of ASD, the senior author prefers to utilize LLIF or ALIF.
ALIF is used at the lumbosacral junction to provide a larger footprint as to minimize subsidence, promote fusion and increase lumbosacral segmental lordosis. Additionally, ALIF allows for decompression of bilateral foramina at the index level, resection of the anterior longitudinal ligament, wide discectomies, and if performed at the L4/5 level there is less mobilization of the psoas and thereby the lumbar plexus (33-35). This may not be feasible pending vascular anatomy, retroperitoneal structures, retroperitoneal scarring, or elevated sacral slope limiting access and graft placement at the L5/S1 level. Additionally, an ALIF can be done in the lateral position allowing for a single-position LLIF and ALIF combination.
In cases with severe kyphotic deformity in need of additional lumbar lordosis, an ACR can be indicated (10). A minimally invasive LLIF with ACR can be performed in lieu of a PSO and achieve 20–30 degrees of sagittal correction with less morbidity than traditional open techniques (9). An ACR supplemented with additional posterior osteotomies can significantly increase segmental lordosis by 72.7% when compared to ACR alone, as described by Turner et al. (36). While ACR is a powerful tool for the minimally invasive surgeon in need of significant sagittal correction, although opportunities may be limited by patient anatomy (i.e., vascular structures or ankylosed levels).
Open osteotomies incur increased risk and morbidity when compared to minimally invasive techniques (37,38). Elevated peri-operative morbidity of open techniques have promoted a mini-open approach for osteotomies (7,8,39). The mini-open PSO in addition to the powerful sagittal correction for ASD. Although long-term studies are needed this technique can be considered maximal sagittal correction when combined with ACR (8).
There are many techniques for thoracolumbar percutaneous pedicle screw placement. Bi-planar fluoroscopic guidance for pedicle screw placement has been described with 2.7% violation of the medial wall with a zero percent complication risk (neurologic or otherwise) (40). Intra-operative navigation has become more commonly used technology for minimally invasive spine surgeons. Innocenzi et al. report a significant increase in accuracy when comparing CT-navigated percutaneous pedicle screw placement versus percutaneous fluoroscopy guided techniques (41). Variations of CT based navigation techniques have been shown to reduce operative time, reduce radiation exposure while improving accuracy compared to traditional fluoroscopic techniques (41-44).
Recent developments in robotic technology have facilitated robotic guidance systems that enable percutaneous placement of pedicle screws. In multilevel constructs, robotic guidance systems deliver pedicle screw accuracy similar to open and fluoroscopic techniques (45-47). However, few studies describe the experience of robotic systems in the setting of minimally invasive spinal reconstruction.
Surgical workflow
Circumferential MISS techniques for ASD offer similar complication and outcome profiles compared to hybrid techniques (22). Variations of staged protocols for circumferential MISS correction of ASD have been described (22,48). All techniques as previously described are utilized as necessary including LLIF, ACR, ALIF, TLIF, facetectomies, and mini-open osteotomies (Figures 1,2).
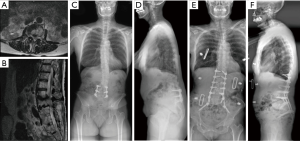
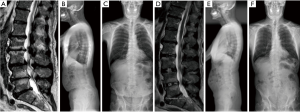
A two-day, staged procedure that combines modular pedicle screw placement, interbody placement, osteotomies, and posterior subfascial rod placement. Stage I consists of the patient placed prone on an open Jackson table with arms extended above the head in preparation for percutaneous pedicle screw placement. The patient is prepped and draped in the standard fashion. Intra-operative imaging is obtained (Figure 3). The technique used for percutaneous pedicle screw placement is left to the discretion of the surgeon. We have found success with biplanar fluoroscopy as well as CT-based navigation. Surgeons should use the technique that provide efficiency and safety regarding pedicle screw placement.
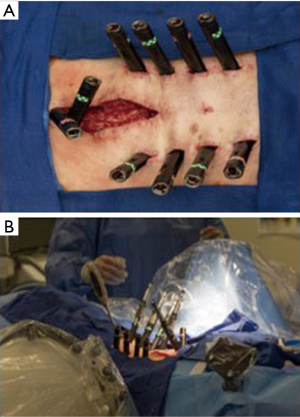
Moreover, we prefer modular pedicle screw placement. Upon placement, the screws are left roughly one centimeter “proud” such that the head can later be capture with a tower through the previously made stab skin incision. The pedicle screws are placed across the thoracic, lumbar and sacral vertebrae. Pelvic fixation is preferred and when performed in a minimally invasive fashion, the S-2 alar-iliac technique allows for easier subfascial rod placement.
It is at this time, when the patient is prone that facetectomies can be performed in a minimally invasive fashion. Facetectomies are performed at any ankylosed segment where sagittal and/or coronal correction is necessary. If significant sagittal correction is needed, the mini-open PSO is also performed at this time.
Following pedicle screw placement and closure of the stab incisions, the patient is turned supine on the surgeon’s choice of table in preparation for an ALIF. With the assistance of an access surgeon an anterior retroperitoneal approach is performed to gain access to the L5/S1 disc space. An appropriately lordotic or hyperlordotic cage is placed dependent on the necessary amount of global and segmental sagittal correction is needed. Although we prefer transpsoas LLIF for the L4−5 interbody placement, an ALIF may be entertained. Contraindications to L4−5 ALIF are primarily vascular in origin (inferior vena cava, aorta and respective bifurcations) or significant retroperitoneal adhesions most commonly from previous surgery. If an ALIF cannot be performed, the TLIF is utilized. An ALIF is preferred due to a larger footprint and lordosis options.
When percutaneous pedicle screw placement and ALIF are complete, the patient is extubated and observed in the intensive care unit. Commonly, a single day (i.e. post-operative day one) of recovery is allowed prior to engaging in the second stage of MISS correction of ASD on post-operative day two. This interval time between staged surgeries is used to resuscitate the patient (if needed) and obtain imaging (CT and standing XR) used to plan for the second stage. Alternatively, success has been had performing the two stages of surgery in sequential days.
Stage II begins with LLIF. The patient is positioned in the lateral decubitus position on a bed with the ability to “break” at the level of the iliac crest. The hips and knees are flexed and axillary and hip roles are placed. The senior author prefers to position with the concavity side of the coronal deformity facing up. This allows for access to the L4−5 disc space as well as providing access to multiple levels via a single or a lesser number of incisions. Furthermore, positioning with the concave side facing up and “breaking” the table can provide correction of the coronal deformity.
LLIF is performed at all requisite levels. Caudal levels are attempted first starting at L4−5 and moving superiorly. The cephalad level is generally ends at T12/L1 or L1−2 for most adult thoracolumbar deformity cases. Intuitively, in ASD cases the disc height is degenerated and can be relatively small compared to healthy disc spaces. As to not promote subsidence, laterally placed interbody height is usually 8−10 mm with varying degrees of lordosis. When significant segmental lordosis is necessary an ACR can be performed with a hyperlordotic cage. ACR in conjunction with osteotomies performed in stage I can be a powerful technique for sagittal correction (36).
When LLIF is complete the patient is transitioned to a prone position again on an open Jackson table. The previously made stab incisions for percutaneous pedicle screw placement are re-opened. The modular screw heads are manually palpated and captured with MISS towers. The screws are advanced to their final seated positions. A computerized rod bending system is used to measure and contour the rod. The rod can be contoured to the native state of the screw heads or varying degrees of correction. The rod is passed in a sub-facial fashion and set screws are sequentially secured.
The post-operative imaging protocol is to obtain immediate CT and MR imaging of the instrumented levels. AP and lateral long cassette XR are obtained on post-operative day one. Outpatient follow-up is scheduled for six-week, twelve-week, six-month and one-year surgical anniversaries with long cassette XR.
Conclusions
Combinations of interbody placement (LLIF, TLIF, ALIF) can be used to obtain sagittal correction. ACR is evolving into a powerful tool for sagittal deformities (10). Additionally, minimally invasive or mini-open techniques can be used to perform osteotomies with less morbidity than open techniques (8,9). MISS techniques are powerful tools for ASD correction and can be tailored on patient-to-patient basis.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Juan S. Uribe is a consultant for NuVasive, Inc. Misonix and SI-Bone. NuVasive, Inc. provides research support and stock options. The other authors have no conflicts of interest to declare.
References
- Banczerowski P, Czigléczki G, Papp Z, et al. Minimally invasive spine surgery: systematic review. Neurosurg Rev 2015;38:11-26; discussion 26. [Crossref] [PubMed]
- Glassman SD, Hamill CL, Bridwell KH, et al. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine 2007;32:2764-70. [Crossref] [PubMed]
- Lafage V, Schwab F, Patel A, et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine 2009;34:E599-606. [Crossref] [PubMed]
- Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine 2003;28:2093-101. [Crossref] [PubMed]
- Smith JS, Shaffrey CI, Klineberg E, et al. Complication rates associated with 3-column osteotomy in 82 adult spinal deformity patients: retrospective review of a prospectively collected multicenter consecutive series with 2-year follow-up. J Neurosurg Spine 2017;27:444-57. [Crossref] [PubMed]
- Yadla S, Maltenfort MG, Ratliff JK, et al. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus 2010;28:E3. [Crossref] [PubMed]
- Fanous AA, Liounakos JI, Wang MY. Minimally Invasive Pedicle Subtraction Osteotomy. Neurosurg Clin N Am 2018;29:461-6. [Crossref] [PubMed]
- Godzik J, Hlubek RJ, de Andrada Pereira B, et al. Combined Lateral Transpsoas Anterior Column Realignment with Pedicle Subtraction Osteotomy to Treat Severe Sagittal Plane Deformity: Cadaveric Feasibility Study and Early Clinical Experience. World Neurosurg 2019;121:e589-95. [Crossref] [PubMed]
- Mundis GM, Turner JD, Kabirian N, et al. Anterior Column Realignment has Similar Results to Pedicle Subtraction Osteotomy in Treating Adults with Sagittal Plane Deformity. World Neurosurg 2017;105:249-56. [Crossref] [PubMed]
- Xu DS, Paluzzi J, Kanter AS, et al. Anterior Column Release/Realignment. Neurosurg Clin N Am 2018;29:427-37. [Crossref] [PubMed]
- Bradford DS, Tay BK, Hu SS. Adult scoliosis: surgical indications, operative management, complications, and outcomes. Spine 1999;24:2617-29. [Crossref] [PubMed]
- Carter OD, Haynes SG. Prevalence rates for scoliosis in US adults: results from the first National Health and Nutrition Examination Survey. Int J Epidemiol 1987;16:537-44. [Crossref] [PubMed]
- Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 2005;30:1082-5. [Crossref] [PubMed]
- Aebi M. The adult scoliosis. Eur Spine J 2005;14:925-48. [Crossref] [PubMed]
- Lowe T, Berven SH, Schwab FJ, et al. The SRS classification for adult spinal deformity: building on the King/Moe and Lenke classification systems. Spine 2006;31:S119-25. [Crossref] [PubMed]
- Schwab F, Farcy JP, Bridwell K, et al. A clinical impact classification of scoliosis in the adult. Spine 2006;31:2109-14. [Crossref] [PubMed]
- Schwab F, Ungar B, Blondel B, et al. Scoliosis Research Society-Schwab adult spinal deformity classification: a validation study. Spine 2012;37:1077-82. [Crossref] [PubMed]
- Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus 2014;36:E6. [Crossref] [PubMed]
- Anand N, Baron EM, Kahwaty S. Evidence basis/outcomes in minimally invasive spinal scoliosis surgery. Neurosurg Clin N Am 2014;25:361-75. [Crossref] [PubMed]
- Pritchett JW, Bortel DT. Degenerative symptomatic lumbar scoliosis. Spine 1993;18:700-3. [Crossref] [PubMed]
- Seo JY, Ha KY, Hwang TH, et al. Risk of progression of degenerative lumbar scoliosis. J Neurosurg Spine 2011;15:558-66. [Crossref] [PubMed]
- Park P, Wang MY, Lafage V, et al. Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J Neurosurg Spine 2015;22:374-80. [Crossref] [PubMed]
- Fontes RB, Fessler RG. Lumbar Radiculopathy in the Setting of Degenerative Scoliosis: MIS Decompression and Limited Correction are Better Options. Neurosurg Clin N Am 2017;28:335-9. [Crossref] [PubMed]
- Cho KJ, Suk SI, Park SR, et al. Short fusion versus long fusion for degenerative lumbar scoliosis. Eur Spine J 2008;17:650-6. [Crossref] [PubMed]
- Phan K, Xu J, Maharaj MM, et al. Outcomes of Short Fusion versus Long Fusion for Adult Degenerative Scoliosis: A Systematic Review and Meta-analysis. Orthop Surg 2017;9:342-9. [Crossref] [PubMed]
- Benglis DM, Elhammady MS, Levi AD, et al. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery 2008;63:191-6. [Crossref] [PubMed]
- Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine 2012;16:329-33. [Crossref] [PubMed]
- Landriel F, Hem S, Yampolsky C. Lateral Transpsoas Approach for Lumbar Indirect Lateral Recess Decompression: 2-Dimensional Operative Video. Oper Neurosurg (Hagerstown) 2019;16:391. [Crossref] [PubMed]
- Navarro-Ramirez R, Berlin C, Lang G, et al. A New Volumetric Radiologic Method to Assess Indirect Decompression After Extreme Lateral Interbody Fusion Using High-Resolution Intraoperative Computed Tomography. World Neurosurg 2018;109:59-67. [Crossref] [PubMed]
- Uribe JS, Vale FL, Dakwar E. Electromyographic monitoring and its anatomical implications in minimally invasive spine surgery. Spine 2010;35:S368-74. [Crossref] [PubMed]
- Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus 2010;28:E9. [Crossref] [PubMed]
- Wang MY. Improvement of sagittal balance and lumbar lordosis following less invasive adult spinal deformity surgery with expandable cages and percutaneous instrumentation. J Neurosurg Spine 2013;18:4-12. [Crossref] [PubMed]
- Bae J, Lee SH. Minimally Invasive Spinal Surgery for Adult Spinal Deformity. Neurospine 2018;15:18-24. [Crossref] [PubMed]
- Bae J, Theologis AA, Strom R, et al. Comparative analysis of 3 surgical strategies for adult spinal deformity with mild to moderate sagittal imbalance. J Neurosurg Spine 2018;28:40-9. [Crossref] [PubMed]
- Dorward IG, Lenke LG, Bridwell KH, et al. Transforaminal versus anterior lumbar interbody fusion in long deformity constructs: a matched cohort analysis. Spine 2013;38:E755-62. [Crossref] [PubMed]
- Turner JD, Akbarnia BA, Eastlack RK, et al. Radiographic outcomes of anterior column realignment for adult sagittal plane deformity: a multicenter analysis. Eur Spine J 2015;24 Suppl 3:427-32. [Crossref] [PubMed]
- Auerbach JD, Lenke LG, Bridwell KH, et al. Major complications and comparison between 3-column osteotomy techniques in 105 consecutive spinal deformity procedures. Spine 2012;37:1198-210. [Crossref] [PubMed]
- Oʼneill KR, Lenke LG, Bridwell KH, et al. Clinical and radiographic outcomes after 3-column osteotomies with 5-year follow-up. Spine 2014;39:424-32. [Crossref] [PubMed]
- Wang MY, Madhavan K. Mini-open pedicle subtraction osteotomy: surgical technique. World Neurosurg 2014;81:843.e11-4. [Crossref] [PubMed]
- Beckman JM, Murray G, Bach K, et al. Percutaneous Minimally Invasive (MIS) Guide Wire-less Self-Tapping Pedicle Screw Placement in the Thoracic and Lumbar Spine: Safety and Initial Clinical Experience: Technical Note. Oper Neurosurg (Hagerstown) 2015;11:530-6. [Crossref] [PubMed]
- Innocenzi G, Bistazzoni S, D’Ercole M, et al. Does Navigation Improve Pedicle Screw Placement Accuracy? Comparison Between Navigated and Non-navigated Percutaneous and Open Fixations. Acta Neurochir Suppl 2017;124:289-95. [Crossref] [PubMed]
- Malham GM, Parker RM. Early experience of placing image-guided minimally invasive pedicle screws without K-wires or bone-anchored trackers. J Neurosurg Spine 2018;28:357-63. [Crossref] [PubMed]
- Siasios ID, Pollina J, Khan A, et al. Percutaneous screw placement in the lumbar spine with a modified guidance technique based on 3D CT navigation system. J Spine Surg 2017;3:657-65. [Crossref] [PubMed]
- Tajsic T, Patel K, Farmer R, et al. Spinal navigation for minimally invasive thoracic and lumbosacral spine fixation: implications for radiation exposure, operative time, and accuracy of pedicle screw placement. Eur Spine J 2018;27:1918-24. [Crossref] [PubMed]
- Ghasem A, Sharma A, Greif DN, et al. The Arrival of Robotics in Spine Surgery: A Review of the Literature. Spine 2018;43:1670-7. [PubMed]
- Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J 2013;22:661-6. [Crossref] [PubMed]
- Hyun SJ, Kim KJ, Jahng TA, et al. Minimally Invasive Robotic Versus Open Fluoroscopic-guided Spinal Instrumented Fusions: A Randomized Controlled Trial. Spine 2017;42:353-8. [Crossref] [PubMed]
- Anand N, Kong C, Fessler RG. A Staged Protocol for Circumferential Minimally Invasive Surgical Correction of Adult Spinal Deformity. Neurosurgery 2017;81:733-9. [Crossref] [PubMed]


