
The use of minimally invasive surgery in spine trauma: a review of concepts
Introduction
Traumatic injuries to the spine can be common in the setting of blunt trauma and delayed diagnosis can have a deleterious effect on patients’ health (1,2). Spine trauma patients, especially poly-trauma patients, can present unique challenges to the spine surgeon (3,4). Spine fractures that require surgical intervention, should be managed promptly to improve or prevent neurologic deficit (5).
Minimally invasive spine surgery (MISS) techniques are valuable treatment modalities for the management of spine trauma patients. Originally used for treatment of degenerative lumbar conditions, MISS presents an alternative to traditional open spine surgery. MISS techniques are based on the preservation of soft tissue, while maintaining the principles of spine decompression, stabilization, and deformity correction. MISS is also a viable treatment option in the context of damage control orthopedics, when patients with multiple traumatic injuries may not be able to tolerate traditional open approaches. In this review, we discuss the different types and classifications of spine trauma, and how minimally invasive techniques can be used in the treatment of these spine injuries. Additionally, we will examine the literature supporting the use of these techniques, while explaining common limitations surgeons may encounter when planning for MISS.
Epidemiology
Spine injuries are common in the setting of blunt trauma. There are over 160,000 estimated spine fractures per year in the United States (1,6). More than 50% of fractures occur at the thoracolumbar (TL) spine T10–L2 with AO-typeA compression fractures, burst (type A3) and wedge compression (type A1), being the most common fracture morphologies (1,7,8). Spine fractures are common in adult males and are associated with high-energy trauma, such as motor vehicle crashes or falls from significant height. Injuries in the elderly population are most likely associated with low-energy trauma such as falls from standing height. One of the most devastating complications of spine fractures is spinal cord injury, which is estimated to occur in 26.5% of TL fractures (7). Additionally, patients with spine trauma can present with multiple traumatic injuries. In patients with TL spine injuries, the rate of concomitant non-contiguous cervical spine was 11%, rate of extremity trauma was 19%, rate of head trauma was 13%, and rate of abdominal trauma was 10% (6,7).
Classification systems
In past decades, the Denis 3-column system was used to classify TL fractures, but its clinical utility was limited, as it did not propose a treatment course or guided decision-making (9,10). Newer schemas base their classification on three components of injury: fracture morphology, neurological status, and integrity of ligamentous structures. The Thoracolumbar Injury Classification and Severity (TLICS) Score (11) and the Subaxial Cervical Spine Injury Classification and Severity (SLIC) Score (12) are widely accepted because they provide a scoring system to guide management (Table 1). Patients with a score of less than four can be managed non-operatively and those with a score of five or more are operative candidates. A score of four is indeterminate and these patients can be managed operatively or non-operatively depending on the surgeon’s clinical decision making.
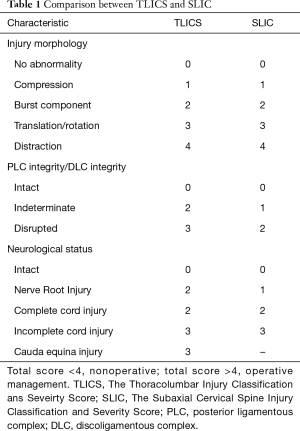
Full table
More recently, the AOSpine Subaxial Cervical Spine and Thoracolumbar Spine Injury Classification Systems were developed. The AOSpine classification systems takes into account fracture morphology, neurological status, and case specific modifiers (8,13). In a meta-analysis comparing the utility of four different classification systems for TL trauma, TLICS was the best system available for guiding therapeutic decision-making in TL spine injuries (9,10). On the other hand, the same study shows that the AOSpine classification system was found to be superior to the TLICS score for classifying fracture morphology with better inter and intraobserver reliability (14). However, additional studies are needed to compare the utility of the newly developed AOSpine TL Spine injury classification system with the TLICS score for making clinical decisions.
Rationale
The main goal of MISS is to reduce approach-associated morbidity, while obtaining similar outcomes as traditional open spine surgery. The treatment goals in spine trauma are to prevent the development of a neurological injury, prevent further neurological damage, provide stability to the spine, and correct post-traumatic deformity. Restoring proper spine alignment enhances neurological recovery and reduces the risk of deterioration of an existing neurological deficit (15). In poly-trauma patients, the principles of damage control orthopedics are often used for surgical decision-making (16-18). The goal of damage control orthopedics is to reduce the physiologic burden and morbidity associated with a traditional open approach, in an unstable, poly-traumatized patient. A retrospective study demonstrated that early surgical stabilization of spine fractures was the only physician-dependent risk factor that was associated with lowering the rate of respiratory failure in poly-trauma patients undergoing thoracic or lumbar surgery (19). Although, poly-trauma patients may benefit from early surgical stabilization, definitive fixation should be delayed until the patient achieves hemodynamic stability and can tolerate physiologically demanding procedure (18).
Minimizing the physiologic burden associated with open procedures is one of the fundamental benefits of MISS (20). In the treatment of type A-compression TL fractures (8), retrospective and prospective studies have shown that MISS approach had decreased blood loss, shorter operative times, and length of stay, when compared to traditional open procedures (21-23). In studies examining post-operative pain, MISS was shown to be beneficial for lowering postoperative pain and improving functional recovery within 3-months of surgery (21).
For single-level TL burst fractures, MISS demonstrated better patient reported outcomes when compared to conservative and open surgical management (24). The morbidity associated with traditional open approaches is a result of the extensive soft tissue dissection that leads to muscle ischemia, denervation, and ultimately, pain (25). Muscle damage eventually causes muscle atrophy and can hinder patients’ rehabilitation capability and overall outcomes (26). This is especially true in the poly-trauma population where an already damaged tissue may benefit from a surgical approach that offers the least risk of approach-associated morbidity (27-30).
MISS is superior to open approach in terms of the infection rate. In a prospective case series, authors reported a 10% infection rate in patients undergoing open operative decompression and internal fixation of TL fractures (31). Reports have shown that surgical site infection (SSI) in MISS procedures ranges from 0.1% in spinal decompression procedures to a 1.5% in spinal fixation and/or fusion procedures, with an overall SSI rate of 0.22% for all spine procedures (32). Compared to a 2–6% infection rate reported for open procedures, MISS is a recommended option in treatment of spine trauma patients to reduced risk of SSI (16,18,27,28,30,32).
Indications
When selecting an optimal surgical approach for the treatment of spine fractures, several factors are taken into consideration: the bony and ligamentous injury pattern, the presence of neurologic injury, the surgeons’ expertise, and the patients’ medical comorbidities and body habitus. TLICS can be used to help guide treatment decision-making, but typically those injuries that require surgery are patients’ who have damage to the posterior ligamentous complex, a neurologic deficit, or a stable compression/burst fractures not amenable for treatment with orthosis (33). MISS techniques have been used in the treatment of unstable fractures with or without spinal cord injury, flexion- and extension-distraction injuries, and unstable sacral fractures (34-36).
One controversy is the need for arthrodesis in the treatment of spine fractures. Instrumentation without fusion is considered for patients with purely bony injuries, such as a transosseous Chance fracture. Studies have shown that non-fusion methods are effective in achieving stability and sagittal alignment, even after removal of implants (37). Hardware can be removed after fracture healing is achieved and confirmed on postoperative CT scan. Otherwise, hardware would be removed if it becomes clinically symptomatic or is causing patient discomfort. A recent meta-analysis found no clear clinical or radiological advantage of fusion in the treatment of burst fractures (38). Additionally, surgical time, blood loss, and maintaining mobility at the fractured level favored the non-fusion group. No difference was established between fusion and non-fusion groups in terms of instrumentation failure, radiological parameters, and pain scores. Therefore, non-fusion methods may be an effective option for the management of TL fractures (37,38).
In a prospective-randomized study comparing fusion to non-fusion in the treatment of TL burst fractures, short segment fixation (SSF) without fusion showed satisfactory results with respect to complications, blood loss and operative time (39). SSF, defined as one level below and one above, has shown to be a valuable option in treatment of TL burst fractures (Figure 1) and fracture-dislocation (40,41). Insertion of a screw at injury level, when allowed by fracture morphology, have been used successfully to provide additional biomechanical support (40,42). Overall, compared with fusion, SSF with MISS has no significant differences with respect to clinical and radiographic outcomes for the treatment of TL burst fractures (23,41,43).
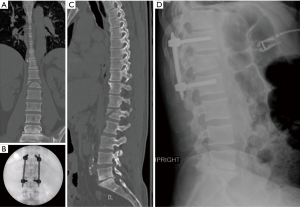
Patients with burst fractures with incomplete neurological deficit classically require anterior column reconstruction. Anterior column support can be obtained through lateral MISS approaches by performing a corpectomy and interbody fusion with the use of expandable titanium cages and anterolateral or pedicle screw fixation (36). Lateral MISS approaches allow for the direct visualization of the pathology and the application of traditional corpectomy techniques, while minimizing approach-associated morbidity (36). Additionally, anterior approaches have resulted in improved neurological outcomes when compared to posterior or lateral decompression techniques (44).
MISS can also be considered for the treatment of flexion-distraction injuries. These fractures may be associated with severe instability due to disruption of posterior stabilizing structures. MISS can provide sufficient stabilization along fracture lines while the healing process occurs (43). A prospective study on patients with flexion distraction injuries showed that there was no difference in the American Spinal Injury Association score and the degree of kyphotic angulation between the MIS and open surgery groups (44). Furthermore, MISS had reduced blood loss and tissue damage compared with open surgical techniques. Similar techniques have also been described in the treatment of extension-distraction injuries in patients with ankylosed spine (45).
Moreover, MISS techniques can be used for the treatment of unstable or complex sacral fractures that require lumbopelvic fixation (LPF). Minimally invasive LPF techniques have been shown to provide adequate biomechanical stability and appropriate fracture reduction for the management of patients with unstable sacral fractures (34,46). Despite several advantages of MISS over traditional open approaches, a spine surgeon can still encounter restrictions or complications in the application of MISS for spine fractures.
The most crucial consideration when attempting MISS in trauma patients is surgical experience in performing the techniques. MISS is associated with a steep learning curve. Because of the reduced tissue exposure in MISS, the lack of visual and tactile anatomic landmarks may present a challenge to the inexperienced surgeon. In a systematic review, a significant reduction in complication rate was demonstrated after a surgeon had performed 30 chronological cases (47). Visualization of anatomic landmarks needs to be achieved with intra-operative fluoroscopy. Therefore, failure to achieve radiographic visualization of key structures is a contraindication for the use of MISS and an open approach should be attempted in such cases.
Additionally, inability to accurately visualize these anatomical structures increases the risk of screw malposition, longer operative times, and radiation exposure (48-50). A study evaluating pedicle screw position with MISS found that 9.7% of the screws were malpositioned. Of the malpositioned screws, 75% were located between L3 and L5 due to poor visualization or interference from the iliac spine (49). In addition, a systematic review showed screw malposition rates ranges between 2.7% and 6.7% for pedicle screws placed under fluoroscopic guidance (51). Inexperience with MISS techniques may often lead to longer operative times, consequently increasing radiation exposure (47). Reported radiation dosage rates for MISS are 10 times higher than traditional open surgery. Therefore, surgical expertise is critical for the application of MISS in patients with spine trauma.
Surgical techniques
Pedicle screw instrumentation
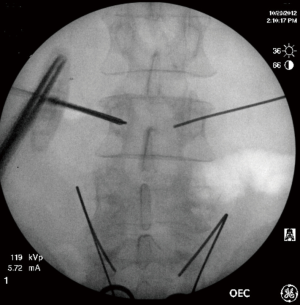
Four different methods have been described for percutaneous placement of pedicle screws: true antero-posterior (AP) targeting, Magerl or Owl’s eye technique (OET), biplanar fluoroscopy and image-guided navigation (52). The preferred method of authors (KE Banagan and SC Ludwig) is the true targeting as it allows for both pedicles to be instrumented simultaneously by two surgeons, minimizing both operative time and radiation exposure (17). A true AP is obtained when anterior and posterior margins are superimposed and only a single superior endplate shadow can be seen (Figure 2). A full description of the true AP targeting method can be found elsewhere (17). This approach reduces the rate of significant radiographic breach to less than 2.9%, and symptomatic breach to near 0% (53,54). Another method for percutaneous pedicle screw insertion is the OET which uses a trajectory down the axis of the pedicle on an oblique view (54). Between the two, the AP targeting technique was associated with a lower risk of facet joint violation in one cadaveric study, when compared to OET (54).
For adequate percutaneous rod placement, screw positioning in both the coronal and sagittal plane is important. If possible, rod passage should start from the rostral end of the construct and should be inserted into the most proximal pedicle screw. Introducing and advancing the rod in the cranial to caudal direction utilizes the shingled morphology of the posterior lamina to protect the spinal cord and neural elements (55). The technique is effective for flexion-distraction injuries with canal retropulsion (Figure 3). Even with the many advantages of the true AP targeting, the use of this technique is contraindicated in patients with poor radiographic visualization due to reduced bone quality or morbid obesity.
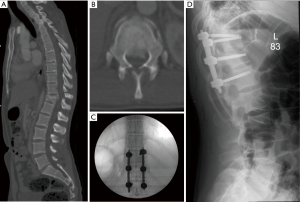
Lateral approach
Some patients may present with anterior column instability and retropulsion into spinal canal, potentially causing neurological injury. Although traditional posterior approaches are the treatment of choice for many spine surgeons, anterior approaches may allow for direct visualization of the ventral elements and removal of spinal compression (42). Anterior decompression, restoration of sagittal alignment, and proper fusion can be achieved with anterior approaches without the need for additional posterior instrumentation. A study comparing anterior-only to posterior-only constructs showed that sufficient maintenance of kyphosis correction can be achieved with anterior instrumentation only (56). Utilizing the lateral approach, anterior column support can be achieved with expandable titanium cages and anterolateral fixation or pedicle screw fixation (36).
Mini-open lateral approaches are alternatives to traditional transthoracic or retroperitoneal approaches, obviating the need for an access surgeon. When compared to a traditional anterior approach, lateral approaches allow for anterior decompression while maintaining the reduced risk for approach-associated morbidity that characterizes MISS techniques (30). Despite the reported success, the most common complication associated with the lateral approach is transient thigh numbness, pain, or weakness (57). This is likely the result of dissection through the psoas major, which can cause trauma to the muscle and potential injury to the lumbar plexus and genitofemoral nerve (58).
Percutaneous vertebral cement augmentation (PVCA)
PVCA techniques include vertebroplasty and kyphoplasty. PVCA techniques are mainly used in treatment of vertebral osteoporotic compression fractures and usually are not employed in the treatment of high-energy TL trauma. The goal of vertebroplasty is to reduce pain by stabilizing the vertebral body and limiting fracture fragment motion. Similarly, kyphoplasty was developed with the aim of reducing deformity from vertebral compression fractures (59). For the latter, a transpedicular approach is used to deliver cement by inserting and inflating a balloon into the vertebral body, to reduce vertebral compression fracture and relieve pressures upon delivery of cement. With this approach, the risk of approach-associated complications is decreased. However, cement-related neurologic injury occurs in <1% of patients (60). Overall, PVCA should be considered in patients with severe osteoporosis who have suffered a vertebral compression fracture, otherwise its role in the treatment of high-energy TL trauma is limited.
MISS in cervical spine
In spine trauma, MISS has largely been limited to the thoracic and lumbar spine. Minimally invasive techniques in the subaxial cervical spine are usually focused on surgical fixation utilizing anterior fixation techniques, thereby avoiding posterior dissection of paraspinal musculature. Clinical and cadaveric studies have demonstrated feasibility of MISS techniques to treat fractures in the atlantoaxial region (61-64). The majority of these techniques are concentrated in atlantoaxial fusion. Feasibility of MISS technique in the placement of C1 lateral mass and C2 pedicle screws using expandable tubular retractors has been reported. A total of six odontoid fractures underwent C1–C2 fusion, achieving solid fusion without motion complications at more than 2-year follow-up (62). However, more clinical and outcomes data is needed to compare its advantage over traditional open cervical approach.
Conclusions
MISS for spine trauma is a valuable option in the treatment armamentarium of spine surgeons. MISS techniques have the potential to reduce open approach-associated morbidity, improve postoperative care and rehabilitation in a variety of spine fractures and clinical scenarios. MISS techniques can even serve as part of a lifesaving damage control algorithm in the treatment of patients with multiple traumatic injuries. The advantages of MISS techniques continue to be a highly investigated topic. Even though the approach offers considerable advantages over open surgery, more outcomes data with a higher level of evidence is needed to prove its true advantage (65). As the field of MISS continue to progress, newer and enhanced techniques will become more readily available for the treatment of spine trauma.
Acknowledgments
None.
Footnote
Conflicts of Interest: SC Ludwig: American Board of Orthopaedic Surgery, Inc., Board or committee member; American Orthopaedic Association, Board or committee member; AO Spine North America Spine Fellowship Support, Research support; ASIP, ISD, Stock or stock Options; Cervical Spine Research Society, Board or committee member; DePuy, A Johnson & Johnson Company, IP royalties, Paid consultant, Paid presenter or speaker; Globus Medical: Paid consultant, Research support; Journal of spinal disorders and techniques, Editorial or governing board; K2M spine, Research support; K2Medical, Paid consultant; OMEGA, Research support; PACIRA, Research support; SMISS, Board or committee member; Synthes, Paid consultant, Paid presenter or speaker; Thieme, QMP, Publishing royalties, financial or material support. The other authors have no conflicts of interest to declare.
References
- Holmes JF, Miller PQ, Panacek EA, et al. Epidemiology of thoracolumbar spine injury in blunt trauma. Acad Emerg Med 2001;8:866-72. [Crossref] [PubMed]
- Reid DC, Henderson R, Saboe L, et al. Etiology and clinical course of missed spine fractures. J Trauma 1987;27:980-6. [Crossref] [PubMed]
- Anderson S, Biros MH, Reardon RF. Delayed Diagnosis of Thoracolumbar Fractures in Multiple-trauma Patients. Acad Emerg Med 1996;3:832-9. [Crossref] [PubMed]
- Harris MB, Sethi RK. The Initial Assessment and Management of the Multiple-Trauma Patient With an Associated Spine Injury. Spine 2006;31:S9-15. [Crossref] [PubMed]
- Cengiz ŞL, Kalkan E, Bayir A, et al. Timing of thoracolomber spine stabilization in trauma patients; impact on neurological outcome and clinical course. A real prospective (rct) randomized controlled study. Arch Orthop Trauma Surg 2008;128:959-66. [Crossref] [PubMed]
- Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine (Phila Pa 1976) 1996;21:492-9. [Crossref] [PubMed]
- Katsuura Y, Osborn JM, Cason GW. The epidemiology of thoracolumbar trauma: A meta-analysis. J Orthop 2016;13:383-8. [Crossref] [PubMed]
- Vaccaro AR, Oner C, Kepler CK, et al. AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976) 2013;38:2028-37. [Crossref] [PubMed]
- Oner FC, Wood KB, Smith JS, et al. Therapeutic decision making in thoracolumbar spine trauma. Spine (Phila Pa 1976) 2010;35:S235-44. [Crossref] [PubMed]
- Yuksel MO, Gurbuz MS, Is M, et al. Is the Thoracolumbar Injury Classification and Severity Score (TLICS) Superior to the AO Thoracolumbar Injury Classification System for Guiding the Surgical Management of Unstable Thoracolumbar Burst Fractures without Neurological Deficit? Turk Neurosurg 2018;28:94-8. [PubMed]
- Lee JY, Vaccaro AR, Lim MR, et al. Thoracolumbar injury classification and severity score: a new paradigm for the treatment of thoracolumbar spine trauma. J Orthop Sci 2005;10:671-5. [Crossref] [PubMed]
- Vaccaro AR, Hulbert RJ, Patel AA, et al. The subaxial cervical spine injury classification system: a novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine (Phila Pa 1976) 2007;32:2365-74. [Crossref] [PubMed]
- Vaccaro AR, Koerner JD, Radcliff KE, et al. AOSpine subaxial cervical spine injury classification system. Eur Spine J 2016;25:2173-84. [Crossref] [PubMed]
- Kaul R, Chhabra HS, Vaccaro AR, et al. Reliability assessment of AOSpine thoracolumbar spine injury classification system and Thoracolumbar Injury Classification and Severity Score (TLICS) for thoracolumbar spine injuries: results of a multicentre study. Eur Spine J 2017;26:1470-6. [Crossref] [PubMed]
- Koreckij T, Park DK, Fischgrund J. Minimally invasive spine surgery in the treatment of thoracolumbar and lumbar spine trauma. Neurosurg Focus 2014;37:E11. [Crossref] [PubMed]
- Banagan K, Ludwig SC. Thoracolumbar Spine Trauma: When Damage Control Minimally Invasive Spine Surgery Is an Option. Semin Spine Surg 2012;24:221-5. [Crossref]
- Gómez JA, Ludwig SC. Minimally Invasive Techniques for Thoracolumbar Spine Trauma. Contemp Spine Surg 2012;13:1-7. [Crossref]
- Tannous O, Shiu B, Koh EY. Minimally invasive spine surgery for thoracolumbar fractures: Damage-control spine stabilization. Semin Spine Surg 2013;25:170-5. [Crossref]
- McHenry TP, Mirza SK, Wang J, et al. Risk factors for respiratory failure following operative stabilization of thoracic and lumbar spine fractures. J Bone Joint Surg Am 2006;88:997-1005. [Crossref] [PubMed]
- Tian F, Tu LY, Gu WF, et al. Percutaneous versus open pedicle screw instrumentation in treatment of thoracic and lumbar spine fractures: A systematic review and meta-analysis. Medicine 2018;97:e12535. [Crossref] [PubMed]
- Jiang XZ, Tian W, Liu B, et al. Comparison of a paraspinal approach with a percutaneous approach in the treatment of thoracolumbar burst fractures with posterior ligamentous complex injury: a prospective randomized controlled trial. J Int Med Res 2012;40:1343-56. [Crossref] [PubMed]
- Reinhold M, Knop C, Beisse R, et al. Operative treatment of 733 patients with acute thoracolumbar spinal injuries: comprehensive results from the second, prospective, internet-based multicenter study of the Spine Study Group of the German Association of Trauma Surgery. Eur Spine J 2010;19:1657-76. [Crossref] [PubMed]
- Wang HW, Li CQ, Zhou Y, et al. Percutaneous pedicle screw fixation through the pedicle of fractured vertebra in the treatment of type A thoracolumbar fractures using Sextant system: an analysis of 38 cases. Chin J Traumatol 2010;13:137-45. [PubMed]
- Kumar A, Aujla R, Lee C. The management of thoracolumbar burst fractures: a prospective study between conservative management, traditional open spinal surgery and minimally interventional spinal surgery. SpringerPlus 2015;4:204. [Crossref] [PubMed]
- Kim DY, Lee SH, Chung SK, et al. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976) 2005;30:123-9. [Crossref] [PubMed]
- Ortega-Porcayo LA, Leal-López A, Soriano-López ME, et al. Assessment of Paraspinal Muscle Atrophy Percentage after Minimally Invasive Transforaminal Lumbar Interbody Fusion and Unilateral Instrumentation Using a Novel Contralateral Intact Muscle-Controlled Model. Asian Spine J 2018;12:256-62. [Crossref] [PubMed]
- Phan K, Rao PJ, Mobbs RJ. Percutaneous versus open pedicle screw fixation for treatment of thoracolumbar fractures: Systematic review and meta-analysis of comparative studies. Clin Neurol Neurosurg 2015;135:85-92. [Crossref] [PubMed]
- Verlaan JJ, Diekerhof CH, Buskens E, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine (Phila Pa 1976) 2004;29:803-14. [Crossref] [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28:S26-35. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and Early Postoperative Complications in Extreme Lateral Interbody Fusion: An Analysis of 600 Cases. Spine 2011;36:26-32. [Crossref] [PubMed]
- Rechtine GR, Bono PL, Cahill D, et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma 2001;15:566-9. [Crossref] [PubMed]
- O'Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 2009;11:471-6. [Crossref] [PubMed]
- Rampersaud YR, Annand N, Dekutoski MB. Use of Minimally Invasive Surgical Techniques in the Management of Thoracolumbar Trauma: Current Concepts. Spine 2006;31:S96-102. [Crossref] [PubMed]
- Jazini E, Weir T, Nwodim E, et al. Outcomes of lumbopelvic fixation in the treatment of complex sacral fractures using minimally invasive surgical techniques. Spine J 2017;17:1238-46. [Crossref] [PubMed]
- Palmisani M, Gasbarrini A, Brodano GB, et al. Minimally invasive percutaneous fixation in the treatment of thoracic and lumbar spine fractures. Eur Spine J 2009;18:71-4. [Crossref] [PubMed]
- Smith WD, Dakwar E, Le TV, et al. Minimally Invasive Surgery for Traumatic Spinal Pathologies: A Mini-Open, Lateral Approach in the Thoracic and Lumbar Spine. Spine 2010;35:S338-46. [Crossref] [PubMed]
- Kim YM, Kim DS, Choi ES, et al. Nonfusion Method in Thoracolumbar and Lumbar Spinal Fractures. Spine 2011;36:170-6. [Crossref] [PubMed]
- Diniz JM, Botelho RV. Is fusion necessary for thoracolumbar burst fracture treated with spinal fixation? A systematic review and meta-analysis. J Neurosurg Spine 2017;27:584-92. [Crossref] [PubMed]
- Wang ST, Ma HL, Liu CL, et al. Is fusion necessary for surgically treated burst fractures of the thoracolumbar and lumbar spine? a prospective, randomized study. Spine 2006;31:2646-52. [Crossref] [PubMed]
- Gelb D, Ludwig S, Karp JE, et al. Successful treatment of thoracolumbar fractures with short-segment pedicle instrumentation. J Spinal Disord Tech 2010;23:293-301. [Crossref] [PubMed]
- Yang H, Shi JH, Ebraheim M, et al. Outcome of thoracolumbar burst fractures treated with indirect reduction and fixation without fusion. Eur Spine J 2011;20:380-6. [Crossref] [PubMed]
- Walker CT, Xu DS, Godzik J, et al. Minimally invasive surgery for thoracolumbar spinal trauma. Ann Transl Med 2018;6:102. [Crossref] [PubMed]
- Dai LY, Jiang LS, Jiang SD. Posterior short-segment fixation with or without fusion for thoracolumbar burst fractures. a five to seven-year prospective randomized study. J Bone Joint Surg Am 2009;91:1033-41. [Crossref] [PubMed]
- Grossbach AJ, Dahdaleh NS, Abel TJ, et al. Flexion-distraction injuries of the thoracolumbar spine: open fusion versus percutaneous pedicle screw fixation. Neurosurg Focus 2013;35:E2. [Crossref] [PubMed]
- Sedney CL, Daffner SD, Obafemi-Afolabi A, et al. A Comparison of Open and Percutaneous Techniques in the Operative Fixation of Spinal Fractures Associated with Ankylosing Spinal Disorders. Int J Spine Surg 2016;10:23. [Crossref] [PubMed]
- Williams SK, Quinnan SM. Percutaneous Lumbopelvic Fixation for Reduction and Stabilization of Sacral Fractures With Spinopelvic Dissociation Patterns. J Orthop Trauma 2016;30:e318-24. [Crossref] [PubMed]
- Sclafani JA, Kim CW. Complications Associated With the Initial Learning Curve of Minimally Invasive Spine Surgery: A Systematic Review. Clinical Orthopaedics and Related Research 2014;472:1711-7. [Crossref] [PubMed]
- Heintel TM, Berglehner A, Meffert R. Accuracy of percutaneous pedicle screws for thoracic and lumbar spine fractures: a prospective trial. Eur Spine J 2013;22:495-502. [Crossref] [PubMed]
- Raley DA, Mobbs RJ. Retrospective Computed Tomography Scan Analysis of Percutaneously Inserted Pedicle Screws for Posterior Transpedicular Stabilization of the Thoracic and Lumbar Spine: Accuracy and Complication Rates. Spine 2012;37:1092-100. [Crossref] [PubMed]
- Rampersaud YR, Foley KT, Shen AC, et al. Radiation Exposure to the Spine Surgeon During Fluoroscopically Assisted Pedicle Screw Insertion. Spine 2000;25:2637-45. [Crossref] [PubMed]
- Court C, Vincent C. Percutaneous fixation of thoracolumbar fractures: current concepts. Orthop Traumatol Surg Res 2012;98:900-9. [Crossref] [PubMed]
- Harris EB, Massey P, Lawrence J, et al. Percutaneous techniques for minimally invasive posterior lumbar fusion. Neurosurg Focus 2008;25:E12. [Crossref] [PubMed]
- Park DK, Thomas AO, St Clair S, et al. Percutaneous lumbar and thoracic pedicle screws: a trauma experience. J Spinal Disord Tech 2014;27:154-61. [Crossref] [PubMed]
- Tannous O, Jazini E, Weir TB, et al. Facet Joint Violation During Percutaneous Pedicle Screw Placement: A Comparison of Two Techniques. Spine (Phila Pa 1976) 2017;42:1189-94. [Crossref] [PubMed]
- Wang MY, Anderson DG, Ludwig SC, et al. Handbook of Minimally Invasive and Percutaneous Spine Surgery. St. Louis: CRC Press, 2011.
- Sasso RC, Renkens K, Hanson D, et al. Unstable thoracolumbar burst fractures: anterior-only versus short-segment posterior fixation. J Spinal Disord Tech 2006;19:242-8. [Crossref] [PubMed]
- Patel VC, Park DK, Herkowitz HN. Lateral transpsoas fusion: indications and outcomes. ScientificWorldJournal 2012;2012:893608. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373:1016-24. [Crossref] [PubMed]
- Savage JW, Schroeder GD, Anderson PA. Vertebroplasty and kyphoplasty for the treatment of osteoporotic vertebral compression fractures. J Am Acad Orthop Surg 2014;22:653-64. [Crossref] [PubMed]
- Bodon G, Patonay L, Baksa G, et al. Applied anatomy of a minimally invasive muscle-splitting approach to posterior C1–C2 fusion: an anatomical feasibility study. Surg Radiol Anat 2014;36:1063-9. [Crossref] [PubMed]
- Holly LT, Isaacs RE, Frempong-Boadu AK. Minimally invasive atlantoaxial fusion. Neurosurgery 2010;66:193-7. [Crossref] [PubMed]
- Belen D, Simsek S. Minimally invasive atlantoaxial fixation with a polyaxial screw-rod construct: technical case report. Neurosurgery 2007;61:E1340. [Crossref] [PubMed]
- Taghva A, Attenello FJ, Zada G, et al. Minimally Invasive Posterior Atlantoaxial Fusion: A Cadaveric and Clinical Feasibility Study. World Neurosurg 2013;80:414-21. [Crossref] [PubMed]
- Oh T, Scheer JK, Fakurnejad S, et al. Minimally invasive spinal surgery for the treatment of traumatic thoracolumbar burst fractures. J Clin Neurosci 2015;22:42-7. [Crossref] [PubMed]


