
Assessment of a rabbit posterolateral spinal fusion using movement between vertebrae: a modification of the palpation exam for quantifying fusions
Introduction
Although rabbit spinal fusions have been attempted since the 1970s, the modern posterolateral rabbit spinal fusion model was first designed by Boden et al. (1-3). Within the Boden et al. study, fusions were assessed using both tensile strength measurements with an Instron electromechanical testing system as well as a manual palpation exam. The manual palpation results, were found to be equivalent to the formal tensile strength testing. Subsequent studies have also confirmed the equivalency of the manual palpation test to biomechanical testing (4-6). As a result, the standard for assessment of posterolateral fusion has become the manual palpation exam.
Given the subjective nature of this exam, however, there have been attempts to better quantify how a fused spine behaves compared to a non-fused spine. Imaging has been used to validate whether or not a vertebral segment has been fused, but radiographic assessment can be very subjective (5). Another biomechanical study observed stiffness at a fused segment using four point bending and determined that there were differences in bending based on whether or not a fusion did occur (7). In a similar study looking at the rat fusion model, fusion was assessed using in vivo and in vitro bending imaging of the spine in order to quantify fusion status (8). All of these studies utilized significant resources (radiographs, Instron machine, etc.) in order to measure fusion status.
Within our experiment we attempted to quantify movement between rabbit vertebrae in fused and non-fused rabbit spines by measuring the angles formed between vertebrae. Our goal was to create a test in which a picture of a rabbit spine could be taken at various angles of flexion/extension. Fusion would be assessed by the change in angle between spinous processes at the level of interest compared to adjacent levels. This could represent a low-cost, reliable objective test for determining fusion status within the rabbit spine. By quantifying the palpation test it would also be easier for future researchers to perform more in-depth statistical analysis to determine differences in fusion techniques in rabbits.
Methods
All experiments were conducted following approval by our Institutional Animal Care and Use Committee (IACUC). Ten 1-year old New Zealand White Rabbits were included. Our surgical technique to achieve posterolateral fusion was based upon a validated and established model previously reported (1). The area in and around the right and left transverse processes were exposed using a standard posterolateral approach between the longissimus and multifidus muscles on both the right and left side. The rabbits were then divided into three groups. Four rabbits were placed in the bilaterally fused group, four rabbits were placed in the unilaterally fused group, and two rabbits were used as a sham or control surgery for the experiment. For the bilateral group, both the right side and left side transverse processes were decorticated and bone graft from the iliac crest was placed at the fusion bed. For the unilateral group, only the left sided transverse processes were decorticated and bone graft from the iliac crest was only placed on the left side. For the sham surgery group, both the right and left side transverse processes were exposed. No decortication or bone graft would be placed between the transverse processes for the sham surgery rabbits.
The fusion mass was assessed using radiographic imaging, and the manual palpation exam. The radiographs were assessed using a 1–3 scale as previously described in the literature (9). Bridging trabeculae and absence of any areas of lucency between transverse processes were used as criteria for grading X-rays as fused. The manual palpation exam was used to grade fusions as either fused or not fused. The radiographs and the manual palpation of the spine allowed us to determine the level of fusion (i.e., either L4/L5 vs. L5/L6). For the manual palpation of the spine, we first euthanized our rabbits using standard techniques. We then stripped a significant amount of soft tissue from the rabbit spine. Soft tissue was removed between vertebrae for all specimens. A thin cuff of soft tissue (approximately 2 mm) was left around the level of interest (i.e., the fusion level) in order to leave the fusion mass undisturbed.
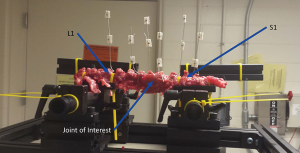
In order to measure the degree and amount of motion at the level of interest, a special device was designed to quantify movement. Figure 1 demonstrates the experimental setup. The yellow plastic straps attach the rabbit spine on to two platforms. The rabbit spine is attached at L1 and S1 as shown in Figure 1. There are silver wires wrapped around the spinous processes and facet joints at the level of interest as well as wires at one and two levels cephalad/caudal to the level of interest. Next silver markers were attached to the silver wires. The setup in Figure 1 is neutral. The flexed position of the spine is shown in Figure 2. Note the yellow protractor resting on the black apparatus. This yellow protractor allowed us to measure the overall angle which we hoped would equal the ideal overall angle. Figure 3 demonstrates the position of the apparatus and spine in extension. Angle measurements were found by triangulating the position of the gray markers during a range of motion using a motion-capture camera.


The performance of the apparatus was first measured by determining the percent error associated with each spine as it went through its range of motion. The overall measured joint angle was formed by the L1 and S1 vertebrae. This was directly compared to the ideal angle. The ideal angle was either 10, 20 or 30 degrees of flexion or extension. This percent error was the (overall angle—ideal overall angle)/ideal overall angle. These performance measurements are provided with enhanced detail in Figure 4.
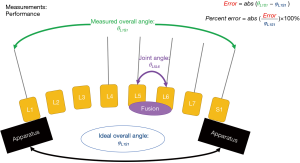
The outcomes measurement was calculated in a similar manner to the performance measurement. The joint angle was measured at the level of fusion (either L5/L6 or L4–L5). This joint angle was compared to the measured overall angle or the angle formed between L1/S1. This percent of overall measured angle equation and the overall experimental setup is further explained in Figure 5.

Statistical analysis
A one-way ANOVA test was used to compare mean percent of overall measured angle between treatment groups. If any results were statistically significant, we performed a post-hoc student’s t-test to determine if there was a statistically significant difference between groups. These P values were adjusted using a Bonferroni correction factor. We used a student’s t-test in order to determine if there was a statistically significant difference between fused and non-fused groups based on palpation exam.
Results
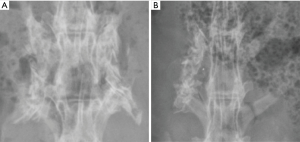
All 10 rabbits completed the experiment. Radiographs for the 5- and 10-week rabbits indicated fusion (3/3 on radiographic fusion scale) in both the unilateral and bilateral fusion groups. Demonstrative radiographs are shown in Figure 6. Manual palpation exam was performed at the attempted level of fusion for the 5- and 10-week cohorts of rabbits. For sham rabbits, a manual palpation exam was performed and indicated no fusion, as expected. For all of the other 8 rabbits, the manual palpation exam indicated a satisfactory fusion.
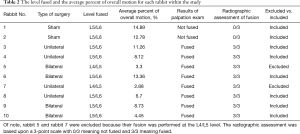
For every rabbit, an attempted fusion was performed at the L5–L6 level. For two rabbits (one in the 5-week group and one in the 10-week group) the fusion occurred at L4–L5. Therefore, given the setup of our design, we could not utilize these rabbits within our study. A summary of the rabbits utilized within this study is included in Table 1.

Full table
Performance
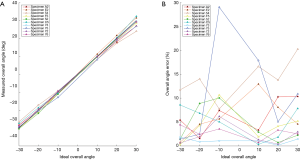
The performance or accuracy of our measuring device was measured for each rabbit during the examination. The results are plotted within two graphs in Figure 7. The graph in Figure 7A shows the ideal overall angle plotted against measured overall angle as measured between L1 and S1. Ideally, there would be a one-to-one ratio, but this was not always the case. The percent error is plotted within Figure 7B. There is a large range in percent error, varying from approximately 2–3% to as much 30%. The majority of data points, however, had error values less than 15%.
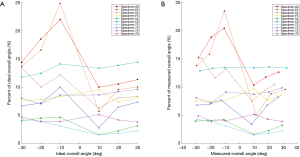
Motion measurements
The motion measurement was obtained at each flexion/extension degree mark for each rabbit. At the joint of interest for fusion (i.e., L5–L6) measurements were taken of the angle between vertebrae. This measurement is plotted against the overall ideal angle and the overall measured angle. These results are shown in Figure 8A,B. These results were combined into an average of measured overall angle. The results are shown in Table 2. When rabbits graded as fused were compared to sham rabbits, there was a trend toward reduction in percent of overall measured angle within the fused group as compared to the sham group (8.77% vs. 13.84%, P=0.14). There was no statistically significant difference in percent of overall measured angle between unilaterally and bilaterally fused groups (8.69% vs. 8.85%, P=0.71).
Full table
Discussion
Our experiment attempted to quantify the motion between vertebral segments within a rabbit spinal fusion model during flexion and extension. By using an ideal overall angle, we were able to flex/extend each spine a defined amount in order to measure the amount of motion at a particular vertebral level of interest (i.e., fusion level). Within our pilot study, we found that there was a trend toward less overall motion at the fused segment as compared to non-fused segments (8.77% vs. 13.84%, P=0.14). There was no statistically significant difference in motion when comparing unilateral and bilaterally fused spines (8.69% vs. 8.85%, P=0.71). These results suggest that measurement of the angle of displacement between vertebrae at various flexion/extension angles may allow researchers to quantify the degree of fusion of a rabbit spine.
The manual palpation exam is a well-established and validated means by which to judge rabbit spine fusion, but obtaining quantitative proof of fusion can be difficult and expensive (3,7,9,10). Unfortunately, what defines motion for non-fusion and fusion definitions is difficult to quantify without the use of a structural testing machine. This equipment may not be easy to access. With future experiments, our model will allow for objective measurement of motion at a level of interest within the rabbit spine. Using only a camera phone, graphical paper, wire and a pencil, an investigator may be able to measure an angle between the L1 and S1 vertebrae at various angles of flexion/extension. During this movement, one might also measure the angle between vertebrae at the level of interest or level of potential fusion. Our hope with future experiments is to better quantify the ratio between the angle involving a potentially fused L5/L6 and the overall measured angle between L1 and S1 that is indicative of a complete fusion. This may reduce the costs associated with an animal experiment (i.e., avoid use of a structural testing machine).
Within our model we did not note any difference in flexion/extension measurements based on whether rabbit spine fusions were unilateral or bilateral. The similar motion in flexion between cohorts is consistent with previous work by Cottrell et al. (7) Furthermore, for those rabbits that were unilaterally fused, there was a fully formed radiographic fusion mass by 5 and 10 weeks on the fused side, these findings suggest that a unilaterally fused rabbit spine is biomechanically equivalent to bilaterally fused rabbit spines.
There are significant limitations to our study. The number of animals was small, and therefore we were not able to reach statistical significance in terms of difference between fused and non-fused segmental motion. Give that this is a pilot study, the authors hope that future studies with larger animal groups might validate this method of measurement of rabbit spinal fusions. We were encouraged that even with this small number of animals we were able to see a trend in terms of difference of motion between rabbit cohorts. During our experimental model we attached wires around the spinous process/facet joints. This may limit motion, and we did not quantify this method of attachment during our experiment. We also did not standardize the amount of soft tissue removed. We attempted to remove as much as possible, including all intertransverse and interspinous ligaments, as well as all volar musculature along the lumbar spine. This, process, however was not strictly controlled. We also did not control for the amount of time between euthanasia of the animal and mechanical testing. This process varied from 30 minutes to 2 hours.
Conclusions
In summary, this is a novel pilot study on defining motion at a potential fusion level in the rabbit spine fusion model. We found a trend toward a smaller ratio of motion at a level of potential fusion (i.e., L5–L6) as compared to a standard overall measured angle (L1–S1). Furthermore, unilateral and bilateral fusions had similar motion at a potential fusion level. Our pilot study offers a potential manner in which to quantify the manual palpation exam for rabbit spinal fusions without the use of a structural testing machine. Future studies will be required to further validate this model.
Acknowledgments
This study was funded in part by the Orthopaedic Research and Education Foundation (OREF).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Animal Care and Use Committee, The Ohio State University.
References
- Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila Pa 1976) 1995;20:412-20. [Crossref] [PubMed]
- Ritsilä V, Alhopuro S. Spinal fusion with free periosteal grafts and its effect on vertebral growth in young rabbits. J Bone Joint Surg Br 1975;57:500-5. [Crossref] [PubMed]
- Ghodasra JH, Daley EL, Hsu EL, et al. Factors influencing arthrodesis rates in a rabbit posterolateral spine model with iliac crest autograft. Eur Spine J 2014;23:426-34. [Crossref] [PubMed]
- Grauer JN, Patel TC, Erulkar JS, et al. 2000 Young Investigator Research Award winner. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine (Phila Pa 1976) 2001;26:127-33. [Crossref] [PubMed]
- Yee AJ, Bae HW, Friess D, et al. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine (Phila Pa 1976) 2004;29:1308-13. [Crossref] [PubMed]
- Riordan AM, Rangarajan R, Balts JW, et al. Reliability of the rabbit postero-lateral spinal fusion model: A meta-analysis. J Orthop Res 2013;31:1261-9. [Crossref] [PubMed]
- Cottrell JM, van der Meulen MC, Lane JM, et al. Assessing the stiffness of spinal fusion in animal models. HSS J 2006;2:12-8. [Crossref] [PubMed]
- Cunningham ME, Beach JM, Bilgic S, et al. In Vivo and In Vitro Analysis of Rat Lumbar Spine Mechanics. Clin Orthop Relat Res 2010;468:2695-703. [Crossref] [PubMed]
- Daffner SD, Waugh S, Norman TL, et al. Effect of serum nicotine level on posterior spinal fusion in an in vivo rabbit model. Spine J 2015;15:1402-8. [Crossref] [PubMed]
- Drespe IH, Polzhofer GK, Turner AS, et al. Animal models for spinal fusion. Spine J 2005;5:209S-216S. [Crossref] [PubMed]


