
Intraoperative ketamine may increase risk of post-operative delirium after complex spinal fusion for adult deformity correction
Introduction
Postoperative delirium is a common surgical complication, affecting more than 2.3 million hospitalized adults and costing the healthcare system approximately $152 billion annually (1). The Center for Medicare and Medicaid Services recently declared delirium a non-reimbursable hospital acquired condition, shifting the burden of cost to hospitals and further incentivizing the identification of predictors of delirium (1). Accordingly, identifying and addressing causes of delirium are a crucial component to improving patient outcomes and reducing healthcare costs in the setting of soaring healthcare expenditures in recent years (2).
Due to a growing elderly population and operational complexity, postoperative delirium is a more common complication after spinal fusion, with an estimated incidence rate of 10% in patients undergoing spinal deformity correction (3). There is growing evidence for pharmacological predictors of postoperative delirium in spinal surgery patients, including opiate analgesics and intra-operative pharmacologic agents including dezocine (3,4). One commonly used intra-operative medication in complex spinal surgery is ketamine, which has been shown to reduce postoperative pain, opioid consumption, and incidence of opiate-induced delirium (5-7). Contrary, there have been other studies that have suggested a contrasting impact with the use of intraoperative ketamine, demonstrating an increased association with postoperative hallucinations (8). To date, despite frequent ketamine administration, the association between intra-operative ketamine administration and post-operative delirium in complex spine surgery remains relatively unknown.
The aim of this study is to determine whether intraoperative ketamine infusion influences the risk of development of postoperative delirium after complex spinal fusion involving ≥5 levels.
Methods
The medical records of 138 adults (≥18 years old) spine deformity patients undergoing elective complex spinal fusion (≥5 levels) for deformity correction at a major academic institution from 2010 to 2015 were reviewed. Institutional review board approval was obtained prior to study initiation (IRB: Pro00066331). Patients were categorized based into two groups based on intraoperative ketamine administration. We identified 98 (71.0%) who had intraoperative ketamine administration and 40 (29.0%) who did not (Ketamine-Use: n=98; No-Ketamine: n=40). Delirium was assessed using the Confusion Assessment Method (CAM) and evaluated as absent or present. The primary outcome investigated in this study was the rate of post-operative delirium.
Baseline characteristics and demographic variables evaluated included patient age, sex, and body mass index (BMI). Comorbidities included depression, anxiety, diabetes, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), coronary artery disease (CAD), atrial fibrillation (A-Fib), history of myocardial infarction (MI), peripheral vascular disease (PVD), hypertension (HTN), hyperlipidemia (HLD), anemia, history of deep vein thrombosis (DVT), history of pulmonary embolism (PE), chronic kidney disease (CKD), and osteoarthritis. Other preoperative variables collected included alcohol use, smoking status, and narcotic use.
Intraoperative variables included number of fusion levels, operative time, estimated blood loss (EBL), administration of packed red blood cell (PRBC) or cell-saver transfusions, and whether a laminectomy and/or osteotomy was performed. Other operative variables assessed included dosage of intra-operative ketamine, use of somatosensory stimulus evoked potentials (SSEP), transcranial motor evoked potentials (TcMEP), electromyography (EMG), and fluoroscopy. Additionally, whether patients received bone graft and intra-operative drain placement were also collected. Intraoperative complications collected included spinal cord injury, nerve root injury, and incidental durotomy.
Postoperative complications included length of stay in hospital (LOS), delirium, fever, urinary tract infection (UTI), ileus, deep and superficial surgical site infection (SSI), wound dehiscence, draining wounds, pneumonia, hypertension (HTN), hypotension, hematoma, anemia, MI, PE, DVT, stroke, sepsis, weakness, sensory deficit, and urinary retention. Rate of unplanned 30-day readmissions were also collected for every patient.
Parametric data were expressed as means ± standard deviation (SD) and compared using the Student’s t-test. Nonparametric data were expressed as median (interquartile range) and compared via the Mann-Whitney U test. Nominal data were compared with the χ2 test. A multivariate nominal logistic regression was used to assess the association between intra-operative ketamine use and post-operative delirium. All tests were two-sided and were statistically significant if the P value was less than 0.05. Statistical analysis was performed using JMP®, Version 13. SAS Institute Inc., Cary, NC, 1989 to 2007.
Results
Patient demographics and preoperative variables
There were 138 adults (≥18 years old) who met the inclusion criteria of this study (No-Ketamine: n=40; Ketamine-Use: n=98; Table 1). There were no significant differences in age, gender, or BMI between the cohorts (No-Ketamine: 27.7±6.6 kg/m2vs. Ketamine-Use: 27.0±6.1 kg/m2, P=0.571; Table 1). The prevalence of comorbidities between the cohorts were similar, including depression (P=0.587), anxiety (P=0.903), diabetes (P=0.319), COPD (P=0.169), CHF (P=0.497), CAD (P=0.623), A-Fib (P=0.497), prior MI (P=0.584), HTN (P=0.424), HLD (P=0.669), anemia (P=0.694), prior DVT (P=0.246), prior PE (P=0.509), CKD (P=0.195), osteoarthritis (P=0.597), alcohol use (P=0.773), current smoking (P=0.787), and pre-operative narcotic use (P=0.764, Table 1).
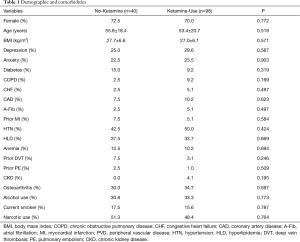
Full table
Intraoperative variable and complications
The median number of fusion levels [No-Ketamine: 9 (range, 7–10) vs. Ketamine-Use: 9 (range, 7–13), P=0.087] and operative time (No-Ketamine: 329.1±128.1 min vs. Ketamine-Use: 329.3±125.0 min, P=0.991) were similar between cohorts (Table 2). The cohort receiving ketamine received a median dose of 49.5±48.4 mg ketamine per operation. There were no significant differences in the other surgical variables including the performance of laminectomy (No-Ketamine: 45.0% vs. Ketamine-Use: 48.9%, P=0.676) or osteotomy (No-Ketamine: 10.0% vs. Ketamine-Use: 22.1%, P=0.098) between the cohorts (Table 2). Both groups had similar intra-operative EBL (No-Ketamine: 1,429.5±1,409.7 mL vs. Ketamine-Use: 1,276.2±1,211.6 mL, P=0.549), PRBC transfusions (No-Ketamine: 47.5% vs. Ketamine-Use: 51.0%, P=0.708), and cell-saver transfusions (No-Ketamine: 60.0% vs. Ketamine-Use: 66.3%, P=0.481; Table 2). The utilization of intraoperative monitoring and imaging were similar, including SSEP (P=0.846), TcMEP (P=0.655), EMG (P=0.223), and fluoroscopy (P=0.438) were similar between cohorts (Table 2). There were no significant differences in intra-operative complications including nerve root or spinal cord injuries, nor incidental durotomy (No-Ketamine: 10.3% vs. Ketamine-Use: 5.1%, P=0.272; Table 2). The proportion of patients receiving bone graft (P=0.605) and having a drain placement (P=0.304) were also similar between the cohorts (Table 2).
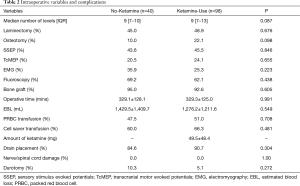
Full table
Postoperative complications
There were no significant differences in overall LOS between the cohorts (No-Ketamine: 7.3±4.5 days vs. Ketamine-Use: 6.5±3.8 days, P=0.323; Table 3). Compared to the No-Ketamine group, the Ketamine-Use cohort experienced a significantly higher incidence of post-operative delirium (No-Ketamine: 2.6% vs. Ketamine-Use: 14.3%, P=0.047). However, rates of unplanned 30-day readmission rates were significantly lower in the cohort receiving intra-operative ketamine (No-Ketamine: 25.0% vs. Ketamine-Use: 9.2%, P=0.014; Table 3). There were no significant differences in the incidence of other post-operative complications including fever (P=0.131), UTI (P=0.788), ileus (P=0.084), deep SSI (P=0.585), wound dehiscence (P=0.844), draining wounds (P=0.880), superficial SSI (P=0.361), pneumonia (P=0.118), HTN (P=0.788), hypotension (P=0.555), hematoma (P=0.873), anemia (P=0.366), MI (P=0.515), PE (P=0.360), DVT (P=0.353), stroke (P=0.360), sepsis (P=0.192), weakness (P=0.435), sensory deficits (P=0.519), and urinary retention (P=0.096, Table 3).

Full table
Multivariate nominal logistic regression analysis
After adjustment for known covariates, older age (OR: 1.171, 95% CI: 1.048–1.307; P=0.005) and receipt of intra-operative ketamine (OR: 9.475, 95% CI: 1.026–87.508, P=0.047) were significant predictors of developing post-operative delirium (Table 4). However, other patient and treatment characteristics including sex, preoperative narcotic use, number of fusion levels, and LOS were not statistically significant predictors of post-operative delirium (Table 4).
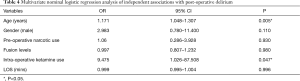
Full table
Discussion
In this retrospective study of patients undergoing spine complex deformity correction surgery involving ≥5 fusion levels, our study demonstrates that the use of intra-operative ketamine is an independent predictor for post-operative delirium.
With an increasing elderly patient population, postoperative delirium is becoming a more common complication (9). Despite the substantial burden it poses to the healthcare system, there is a paucity of data on the incidence and risk factors associated with this complication after spine surgery. In a retrospective study of 578,457 patients who underwent lumbar spine surgery, Fineberg et al. identified an overall incidence rate of postoperative delirium to be 8.4 events per 1,000 (1). Moreover, the authors found that older age (≥65 years old) was an independent predictor for postoperative delirium (1). Similarly, Adogwa et al. identified an incidence rate of postoperative delirium to be 18% in an elderly scoliosis patient cohort undergoing correction surgery (10). In a recent comprehensive meta-analysis, Shi and colleagues, identified six prior studies exploring delirium after spinal surgery (11). Across these studies, the incidence rates of post-operative delirium ranged from 0.84% to 21.3% between studies performed in the U.S. (1), China (4,12), Japan (13,14), and Korea (15). Furthermore, Shi et al. identified that age was significantly associated with postoperative delirium (11). Analogous to the aforementioned studies, our analysis demonstrates an incidence rate of the development of postoperative delirium after complex spine surgery to be 10.9% and corroborates the finding of increasing age being a significant risk factor.
While the majority of risk factors for delirium to date are pre- and postoperative variables, there is a growing evidence that intraoperative and pharmacological variables can also predispose patients to development of postoperative delirium. In the meta-analysis by Shi et al., other significant risk factors for postoperative delirium included length of surgery and amount of intraoperative blood loss (11). Additionally, Jiang et al. extended this analysis to include intraoperative hypotension <80 mmHg and use of dezocine as significant risk factors for postoperative delirium (3,4). Similarly, in a retrospective study of 549 patients who underwent spinal surgery, Gao et al. determined that the number of prescribed medications and opiate analgesic drug used intraoperatively were predictors of postoperative delirium (4). Therefore, with a growing use of various pharmacological agents intraoperatively, there has been an increasing emphasis to identify agents that are associated with inferior outcomes, such as postoperative delirium.
Complex spinal fusion surgeries often involve severe postoperative pain which is commonly treated with narcotic-based pain regimens (16). Due to the increasing opioid epidemic within the U.S., in 2016 the American Pain Society released an updated set of practice guidelines for the management of postoperative pain emphasizing multimodal approaches and minimizing the use of opioids (17). Since that time, there have been many studies demonstrating that intraoperative ketamine infusions are associated with decreased immediate and persistent postoperative pain (18-23). As a result, new guideline recommendations included the administration of a subanesthetic intraoperative bolus dose of ketamine perioperatively in patients undergoing major surgery, including complex spinal surgery (17). In a single institutional study of 147 patients who underwent elective spine surgery, Nielsen et al. demonstrated that the use of intraoperative ketamine was associated with reduction of opioid use and pain at 1 year after (24). With the reduction of pain, our study demonstrated a lower 30-day readmission rate with the administration of intraoperative ketamine, which may be due to the reduction in post-operative pain. Additionally, due to the incidence of opioid-induced delirium, transitioning post-operative pain regimens to include ketamine has been thought to reduce the incidence of opioid-induced delirium (8). In fact, in a small prospective cohort of 58 patients, Hudetz and colleagues demonstrated a 30% reduction in postoperative delirium after cardiac surgery with perioperative ketamine administration (25). However, the administration of intraoperative ketamine is decided by the providing surgical team on an individual basis.
While many post-operative pain regimens across surgical specialties have since transitioned to include ketamine, there is a paucity of evidence attributing delirium-protectant effects of ketamine (8). In a systematic literature review of the association between “ketamine” and “postoperative delirium”, Avidan et al. yielded six studies with two demonstrating decreased delirium, one with an increase in delirium, one with equivocal results, and two without any delirium patients (8). As a result of inconsistent findings throughout the literature, Avidan and colleagues conducted an international, multicenter, double-blind, randomized clinical trial with 672 elderly patients (≥60 years old) undergoing major surgeries (8). The authors concluded that there were no significant differences in the incidence postoperative delirium, postoperative pain, or postoperative opioid usage between the ketamine and control groups (8). In fact, the authors found that patients receiving ketamine tended to have an increased incidence of hallucinations and nightmares (8). This particular finding has been noted previously (5) and is also formally in the American Pain Association’s guidelines for consideration. Ketamine is an NMDA and HCN1 antagonist that lead to dissociative anesthesia that includes hypnosis and analgesia (26). Given the similarity between the hypoactive symptoms, altered mental status, and altered arousal of delirium and the dream-like state of dissociative anesthesia due to ketamine, it is possible that the two mechanisms are associated. While the pathophysiological mechanisms of both delirium and dissociative anesthesia are still debated, the similarity in clinical presentations suggests some degree of related mechanisms. Overall, our study further supports the notion that intraoperative ketamine does not prevent delirium, but may actually be significantly associated with an increase incidence of postoperative delirium after complex spine surgery involving ≥5 fusion levels.
Postoperative delirium is a serious health, social, and economic burden on the healthcare system and has been associated with inferior outcomes and increased complication rates, hospital length of stay, inpatient morbidity and mortality, and healthcare costs (2,27). The estimated cost associated with delirium is more than $150 billion annually and is found to complicate more than 2.3 million adult hospitalizations in the U.S yearly (1,28,29). In the retrospective study by Fineberg et al., the authors found that patients experiencing postoperative delirium after spine surgery were associated with an average cost increase of $13,392, compared to non-delirium patients ($29,970 vs. $16,578, respectively) (1). Additionally, patients experiencing delirium more often require nursing home placement after discharge, and are less likely to maintain compliance with their rehabilitation protocols, causing significant stress to both patients and family members (1). Because there are no true pharmacological treatments for delirium (8), identifying and addressing potential modifiable causes of postoperative delirium may allow for preventative protocols that can be implemented to better overall patient outcomes and reduce soaring healthcare costs.
This study has limitations with potential implications for study interpretation. Although all variables were recorded pre-, peri-, and postoperatively, they were reviewed retrospectively and, as such, are limited by the weaknesses inherent to retrospective analyses. Additionally, the severity of postoperative delirium was not known. Given the prevalence of purely hypoactive symptoms in delirium, this could have introduced a selection bias for the more severe and clinically obvious presentations of delirium. While the CAM tool was used, there may be patients that may not have been included due to inaccurate use of CAM. Additionally, the duration of symptoms was not collected, and therefore are also subject to selection bias. Furthermore, a relatively small patient sample size from only one academic center was used, making broad conclusions difficult and potentially biasing our results for particular patient population or treatment paradigms. Specifically, the impact age has on post-operative delirium and the implications that this may have on our results. Despite these limitations, this study has demonstrated that ketamine serves as an independent risk factor for the development of postoperative delirium. We aim for the results of this study to enable prevention and earlier detection of post-operative delirium after spine surgery, ultimately mitigating the burden of delirium on patients and the healthcare system after spine surgery.
Conclusions
Our study suggests that the intraoperative use of ketamine may increase the risk of post-operative delirium. Preventative measures and early awareness of the risk associated with intraoperative ketamine may help reduce rates of postoperative delirium. Further studies are necessary to understand the physiological effect intraoperative ketamine has on patients undergoing complex spinal fusions in order to better overall patient care and reduce healthcare resources.
Acknowledgements
C. Rory Goodwin, MD, PhD received grants from the Burroughs Wellcome Fund, North Carolina Spine Society, and the NIH/NINDS K12 NRCDP Physician Scientist Award, Robert Wood Johnson Harold Amos Medical Faculty Development Program.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB: Pro00066331). While there is a research consent provided to all patients treated at the academic institution, there is not a written consent from each patient to be reviewed for this study.
References
- Fineberg SJ, Nandyala SV, Marquez-Lara A, et al. Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine (Phila Pa 1976) 2013;38:1790-6. [Crossref] [PubMed]
- Elsamadicy AA, Adogwa O, Lydon E, et al. Depression as an independent predictor of postoperative delirium in spine deformity patients undergoing elective spine surgery. J Neurosurg Spine 2017;27:209-14. [Crossref] [PubMed]
- Jiang X, Chen D, Lou Y, et al. Risk factors for postoperative delirium after spine surgery in middle- and old-aged patients. Aging Clin Exp Res 2017;29:1039-44. [Crossref] [PubMed]
- Gao R, Yang ZZ, Li M, et al. Probable risk factors for postoperative delirium in patients undergoing spinal surgery. Eur Spine J 2008;17:1531-7. [Crossref] [PubMed]
- Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010;113:639-46. [PubMed]
- Pendi A, Field R, Farhan SD, et al. Perioperative Ketamine for Analgesia in Spine Surgery: A Meta-analysis of Randomized Controlled Trials. Spine (Phila Pa 1976) 2018;43:E299-307. [Crossref] [PubMed]
- Mathiesen O, Dahl B, Thomsen BA, et al. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J 2013;22:2089-96. [Crossref] [PubMed]
- Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267-75. [Crossref] [PubMed]
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [Crossref] [PubMed]
- Adogwa O, Elsamadicy AA, Vuong VD, et al. Association between baseline cognitive impairment and postoperative delirium in elderly patients undergoing surgery for adult spinal deformity. J Neurosurg Spine 2018;28:103-8. [Crossref] [PubMed]
- Shi C, Yang C, Gao R, et al. Risk Factors for Delirium After Spinal Surgery: A Meta-Analysis. World Neurosurg 2015;84:1466-72. [Crossref] [PubMed]
- Li H, Li CD, Yi XD, et al. Analysis of risk factors for delirium in the elderly patients after spinal operation. Beijing Da Xue Xue Bao Yi Xue Ban 2012;44:847-50. [PubMed]
- Ushida T, Yokoyama T, Kishida Y, et al. Incidence and risk factors of postoperative delirium in cervical spine surgery. Spine (Phila Pa 1976) 2009;34:2500-4. [Crossref] [PubMed]
- Kawaguchi Y, Kanamori M, Ishihara H, et al. Postoperative delirium in spine surgery. Spine J 2006;6:164-9. [Crossref] [PubMed]
- Lee JK, Park YS. Delirium after spinal surgery in Korean population. Spine (Phila Pa 1976) 2010;35:1729-32. [Crossref] [PubMed]
- Bajwa SJ, Haldar R. Pain management following spinal surgeries: An appraisal of the available options. J Craniovertebr Junction Spine 2015;6:105-10. [Crossref] [PubMed]
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131-57. [Crossref] [PubMed]
- Abu-Shahwan I. Ketamine does not reduce postoperative morphine consumption after tonsillectomy in children. Clin J Pain 2008;24:395-8. [Crossref] [PubMed]
- Dal D, Celebi N, Elvan EG, et al. The efficacy of intravenous or peritonsillar infiltration of ketamine for postoperative pain relief in children following adenotonsillectomy. Paediatr Anaesth 2007;17:263-9. [Crossref] [PubMed]
- Elhakim M, Khalafallah Z, El-Fattah HA, et al. Ketamine reduces swallowing-evoked pain after paediatric tonsillectomy. Acta Anaesthesiol Scand 2003;47:604-9. [Crossref] [PubMed]
- Laskowski K, Stirling A, McKay WP, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 2011;58:911-23. [Crossref] [PubMed]
- O'Flaherty JE, Lin CX. Does ketamine or magnesium affect posttonsillectomy pain in children? Paediatr Anaesth 2003;13:413-21. [Crossref] [PubMed]
- McNicol ED, Schumann R, Haroutounian S. A systematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain. Acta Anaesthesiol Scand 2014;58:1199-213. [Crossref] [PubMed]
- Nielsen RV, Fomsgaard JS, Nikolajsen L, et al. Intraoperative S-ketamine for the reduction of opioid consumption and pain one year after spine surgery: A randomized clinical trial of opioid-dependent patients. Eur J Pain 2019;23:455-60. [Crossref] [PubMed]
- Hudetz JA, Patterson KM, Iqbal Z, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009;23:651-7. [Crossref] [PubMed]
- Sleigh J, Harvey M, Voss L, et al. Ketamine – More mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care 2014;4:76-81. [Crossref]
- Elsamadicy AA, Wang TY, Back AG, et al. Post-operative delirium is an independent predictor of 30-day hospital readmission after spine surgery in the elderly (≥65years old): A study of 453 consecutive elderly spine surgery patients. J Clin Neurosci 2017;41:128-31. [Crossref] [PubMed]
- Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc 2012;13:818.e1-10. [Crossref] [PubMed]
- Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168:27-32. [Crossref] [PubMed]


