
Chordoma of the mobile spine and sacrum: clinical management and prognosis
Introduction
Chordomas were first described by Virchow in 1857 and their origin from the notochord were first reported by Müller in 1858 (1). Chordomas are rare, locally aggressive tumors that account for 1–4% of all primary bone tumors. They often recur locally and can metastasize (2,3). Data from the Surveillance, Epidemiology, and End Results (SEER) program revealed an annual incidence of 0.08 per 100,000 populations (3). Additionally, chordomas are more common in men and rare in blacks and patients younger than 40 (3). Approximately 32–40% of chordomas are cranial, 10–33% spinal, and 29–50% sacral (3,4). Within the mobile spine, the most common location for a chordoma varies. Reported frequency for each location in the mobile spine includes lumbar spine (35–57%), cervical spine (29–48%) and thoracic spine (13–17%) (5-7).
Treatment options for chordomas include surgical resection, radiotherapy, and chemotherapy. Radiotherapy is most often used in patients with local recurrence, while chemotherapy is usually reserved for patients with metastasis (8). Surgical resection is associated with a significant difference in survival compared to those who do not undergo surgery (9). En bloc surgical resection has been the optimal treatment modality since it was first introduced by Stener and Gunterberg in the 1970s (10). In 2016, Lee et al., using the SEER database, found that the overall survival of 1,593 patients was 61% at five years and 41% at 10 years (11).
The purpose of our study was to report a single center’s experience with the operative and non-operative management outcomes of spinal and sacral chordomas over a 23-year period.
Methods
With approval from our institutional review board, we evaluated our institution’s pathology database from 1994 to 2016 to identify patients diagnosed with chordomas. Inclusion criteria were chordomas of the mobile spine and sacrum. Exclusion criteria were chordomas of the clivus and resection performed at another institution. Of the 18 patients diagnosed with chordomas at our institution, 12 met our inclusion criteria. Four were excluded for chordomas in the clivus and two presumably were treated elsewhere. From these 12 patients, we collected patient demographics (age, gender, and race) as well as the type of resection performed (en bloc, piecemeal), complications, and recurrence. There were four females and eight males with an average age of 64 [32–87] years. All of the patients were Caucasian. The average follow-up period was 60 (range, 3–161) months.
Statistical analysis
Fischer exact test was used to test for significance between categorical variables with significance set at P<0.05.
Results
Presenting symptoms
Nine of the 12 patients presented with pain localized to the site of the lesion. The average time from pain onset to diagnosis was 17 (range, 0–108) months. One of the nine patients who presented with pain initially had back pain three years earlier. At that time, an MRI was performed demonstrating a herniated disc at the L4–L5 level. He completed physical therapy and received an epidural block which resulted in complete resolution of his symptoms. Three years later, this patient was lifting at work and had acute onset back pain at which time a MRI revealed a mass on the posterior aspect of L4. One patient presented to the emergency department with two months of progressive lower extremity weakness and numbness with difficulty walking. Another patient had 15 months of neurogenic bowel and bladder problems along with back pain. One patient was diagnosed on presentation to the emergency department following imaging for a fall. This patient was asymptomatic, but a mass was noted on CT and further workup led to the diagnosis of chordoma.
Lesion location
Of the 12 lesions included in this analysis, seven were in the sacrum and five in the mobile spine (one cervical, two thoracic, two lumbar).
Surgical management and outcomes
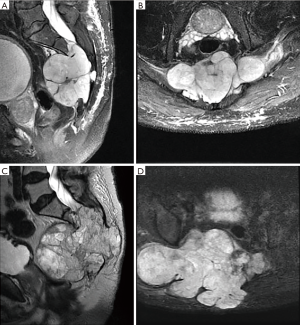
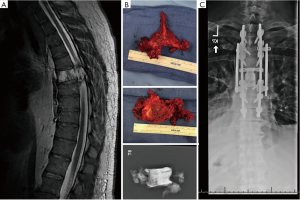
Ten of the 12 patients managed at this Regional Cancer Center were treated surgically. Four of the surgical patients were managed by orthopaedic surgeons, one by a combined orthopaedic/neurosurgery team, and five by neurosurgeons. Of the two who elected not to have surgery, one had a large sacral lesion and multiple medical co-morbidities that made en bloc excision not feasible and received chemotherapy instead (Figure 1) and the other, also with a sacral lesion, opted out of any/all treatment. Of the 10 patients treated surgically, six underwent en bloc resection. Of the five patients with chordoma of the mobile spine, two underwent total en bloc spondylectomy (TES), as previously described (12-14) (Figures 2,3).
Radiation treatment
In this series, most patients (eight of 12) underwent postoperative radiation therapy. Three of the eight patients given conventional postoperative radiation received a total dose of fractioned radiation greater than 60 Gy. Two patients received conventional fractioned radiation totaling less than 60 Gy. One patient was treated with a one-time tomotherapy dose of 15 Gy. Another patient was treated with 75.6 Gy of fractioned postoperative external beam and proton beam radiation therapy at another academic medical center. The final patient receiving post-operative radiation therapy was treated at an outside hospital and the treatment summary is no longer available. One additional patient received 50.4 Gy pre-operative radiation and postoperative SBRT only to bilateral adrenal masses.
Complications
Intraoperative complications included cardiac arrest, pleural tear, and excessive blood loss (8 L). One patient had failure of the anterior posterior spine fusion and underwent a second surgery for a revision fusion. Additionally, three patients with sacral chordomas had wound complications. One patient was diagnosed with a wound infection 13 days after surgery. Escherichia coli and Klebsiella pneumoniae grew in the cultures along with Peptostreptococcus species and Gamma strep not enterococcus. This patient received preoperative radiation treatment totaling 50.4 Gy in 1.8 Gy fractions. The second patient with a postoperative wound infection underwent incision and drainage at an outside hospital following a second surgery for chordoma recurrence. This patient is still being managed for wound dehiscence and failure to heal secondary to radiation therapy 18 months after the second surgery. The third patient had wound dehiscence and was treated as an outpatient. The rate of wound complications in the sacral group as compared to the mobile spine group did not reach statistical significance (P=0.2).
Recurrence and mortality
One patient with piecemeal resection had a recurrence and one patient with incomplete resection has postoperative metastatic lesions to the liver and lung. However, the recurrence rate between piecemeal versus en bloc resections did not reach statistical difference (P=0.13). Of the two patients who elected non-surgical management, one declined all treatment and is currently alive 171 months following diagnosis (age of 95); the second had a non-resectable tumor and was treated with Gleevec for seven years. He declined radiation treatment and died 96 months following diagnosis. A third patient died from complications of acute myeloid leukemia 52 months after chordoma resection. Two other patients died 3 and 120 months after surgery of conditions not directly related to the chordoma, but we were unable to find out what these were.
Discussion
We report our experience with the surgical management of chordomas of the mobile spine and sacrum over a 23-year period at our institution. Our institution’s gender distribution of four females and eight males is consistent with other studies’ findings of chordomas occurring twice as frequently in men as compared with women (15,16). Additionally, all twelve of the patients in our study were Caucasian which is consistent with a rare incidence of chordomas in patients of black African descent (15).
Presentation
Pain is the most common presenting symptom of chordomas (7,17,18). Patients may also present with neurologic symptoms if the mass compresses the spinal cord or nerve roots. In the sacrum, nerve root involvement can manifest changes in bowel and bladder including incontinence. In our 12 patients with known presentation, the average time from symptom onset to diagnosis was 17 months. This is consistent with other literature, which reports mild symptoms of pain present for 12 to 24 months before the establishment of diagnosis (16).
Lesion location
In this series, the sacrum was the most common location. This is consistent with other studies where the sacrococcygeal location is the most common anatomic location for chordomas (4). While other studies have reported the cervical spine to be the most common location within the mobile spine, our study of twelve patients had only one tumor in the cervical spine and two each in the thoracic and lumbar regions (5,7).
Management
En bloc surgical resection with a circumferential margin of uninvolved tissue has been considered standard initial management of chordomas if they are surgically resectable (4,19). Ahmed et al. found 10-year survival rates of 88% and 31% in patients who had undergone total resection compared to subtotal resection (17). Additionally, Shah et al. recently round that TES for spinal tumors, including chordomas, resulted in only a 6.3% tumor recurrence rate (20). Using their technique of a two-stage modified en bloc spondylectomy employing threadwire, a negative margin rate of 94% was achieved (20).
New data suggests that en bloc resection may not be necessary if intralesional debulking and separation surgery are followed by stereotactic body radiation (21). Although, this is a single institution study and additional follow up and reproducibility of the results from other centers is needed. Photon or proton beam radiation for recurrent or residual disease is also standard albeit only a few institutions have these technologies (4). In this series most patients underwent post-operative fractioned conventional radiation therapy of higher than 60 Gy.
Other management options include radiation only and/or chemotherapy. However, chordomas have generally been considered resistant to ionizing radiation and chemotherapy (4). Recent advances in photon and proton radiation therapy, as well as directed targeting with monoclonal antibodies, may lead to improved outcomes for patients who are poor surgical candidates or those with local recurrence (4).
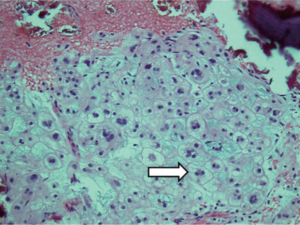
The use of targeted therapy for chordoma treatment has arisen as the molecular pathways involved in chordoma formation have been identified. Brachyury is a transcription factor that is essential for notochord differentiation (22). It encodes a gene needed for development of posterior mesodermal elements but is not expressed in mature tissue. However, chordomas have been shown to be consistently positive for immunoreaction to brachyury (22). Additionally, brachyury gene duplication is often seen in familial chordomas (22). Otani et al. found that brachyury was expressed in all (23) chordomas patients (n=27), albeit with variable levels of expression. Patients with tumors expressing high levels of brachyury were found to have significantly shorter progression free survival compared to those with lower brachyury expression tumors (23). The molecular findings in a chordoma may change how we think about a patient’s prognosis. Bettegowda et al. found the A variant of single nucleotide polymorphism (SNP) rs2305089 in the brachyury gene present in 102 of 109 patients. The patients with this A variant had significantly improved survival compared to those lacking the variant (24).
Recent work has also shown that chordomas often express receptor tyrosine kinases (RTKs) and variation in epidermal growth factor receptor (EGFR) gene copy numbers making RTKs and EGFRs possible targets in the treatment of chordomas (22). Platelet-derived growth factor-alpha and platelet-derived growth factor-beta are RTKs that have been shown to be over expressed and over activated in chordomas (22). This led to the use of imatinib, a PDGFR inhibitor, in the treatment of chordomas. Hindi et al. reported on the use of imatinib in the treatment of 48 consecutive patients with inoperable chordomas. This study found 74% of patients had stable disease for almost 10 months and 21% of patients were progression-free at 18 months (25).
Lesion recurrence and complications
Incidence of reported local recurrence for chordomas ranges from 66% to 75% (26,27). Boriani et al. found that the only treatment protocol associated with continuously disease-free survival at follow-up longer than five years was margin-free en bloc resection (5). Additionally, patients with sacral chordomas have a poor prognosis compared to those with chordomas of the mobile spine (17).
Study limitations
The limitations of our study are the small sample size and length of study span. The small sample size is best explained by the rarity of these tumors. While there are studies with longer time spans, the surgical management techniques, as well as imaging modalities, have changed significantly over this time, making comparison of treatment outcomes less reliable. Although significance difference in recurrence rates was not found between piecemeal versus en bloc resection we attribute this to the small sample size and continue to advocate for en bloc resection when feasible.
Additionally, this review was performed post-transition to electronic medical records, limiting available patient information to that which was scanned. Lastly, patient reported outcomes were not collected for most patients in this study. While patient reported outcomes for chordomas and other primary spine tumors are still being defined, van Wulfften et al. recently published a revised sacral tumor survey that may be appropriate to use in patients with sacral chordomas going forward (28).
Conclusions
This series identified a lower recurrence rate in patients managed with en bloc resection as opposed to piece meal or intralesional resection. Sacral chordoma patients had higher wound complication rates as compared to chordomas of the mobile spine. The long life expectancy of non-surgically managed patients underscores the indolent nature of chordomas.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional review board.
References
- Friedmann I, Harrison DF, Bird ES. The fine structure of chordoma with particular reference to the physaliphorous cell. J Clin Pathol 1962;15:116-25. [Crossref] [PubMed]
- Bailey CS, Fisher CG, Boyd MC, et al. En bloc marginal excision of a multilevel cervical chordoma. Case report. J Neurosurg Spine 2006;4:409-14. [Crossref] [PubMed]
- McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control 2001;12:1-11. [Crossref] [PubMed]
- Sciubba DM, Chi JH, Rhines LD, et al. Chordoma of the spinal column. Neurosurg Clin N Am 2008;19:5-15. [Crossref] [PubMed]
- Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976) 2006;31:493-503. [Crossref] [PubMed]
- Gokaslan ZL, Zadnik PL, Sciubba DM, et al. Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine 2016;24:644-51. [Crossref] [PubMed]
- Bjornsson J, Wold LE, Ebersold MJ, et al. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer 1993;71:735-40. [Crossref] [PubMed]
- Pham M, Awad M. Outcomes following surgical management of cervical chordoma: A review of published case reports and case series. Asian J Neurosurg 2017;12:389-97. [Crossref] [PubMed]
- Zhou H, Jiang L, Wei F, et al. Chordomas of the upper cervical spine: clinical characteristics and surgical management of a series of 21 patients. Chin Med J (Engl) 2014;127:2759-64. [PubMed]
- Stener B, Gunterberg B. High amputation of the sacrum for extirpation of tumors. Principles and technique. Spine (Phila Pa 1976) 1978;3:351-66. [Crossref] [PubMed]
- Lee IJ, Lee RJ, Fahim DK. Prognostic Factors and Survival Outcome in Patients with Chordoma in the United States: A Population-Based Analysis. World Neurosurg 2017;104:346-55. [Crossref] [PubMed]
- Mesfin A, El Dafrawy MH, Jain A, et al. Total En Bloc Spondylectomy for Primary and Metastatic Spine Tumors. Orthopedics 2015;38:e995-e1000. [Crossref] [PubMed]
- Kawahara N, Tomita K, Murakami H, et al. Total en bloc spondylectomy of the lower lumbar spine: a surgical techniques of combined posterior-anterior approach. Spine (Phila Pa 1976) 2011;36:74-82. [Crossref] [PubMed]
- Kawahara N, Tomita K, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: surgical techniques and related basic background. Orthop Clin North Am 2009;40:47-63. vi. [Crossref] [PubMed]
- Anson KM, Byrne PO, Robertson ID, et al. Radical excision of sacrococcygeal tumours. Br J Surg 1994;81:460-1. [Crossref] [PubMed]
- Baratti D, Gronchi A, Pennacchioli E, et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol 2003;10:291-6. [Crossref] [PubMed]
- Ahmed R, Sheybani A, Menezes AH, et al. Disease outcomes for skull base and spinal chordomas: a single center experience. Clin Neurol Neurosurg 2015;130:67-73. [Crossref] [PubMed]
- Sundaresan N, Huvos AG, Krol G, et al. Surgical treatment of spinal chordomas. Arch Surg 1987;122:1479-82. [Crossref] [PubMed]
- York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 1999;44:74-9; discussion 9-80. [Crossref] [PubMed]
- Shah AA, Paulino Pereira NR, Pedlow FX, et al. Modified En Bloc Spondylectomy for Tumors of the Thoracic and Lumbar Spine: Surgical Technique and Outcomes. J Bone Joint Surg Am 2017;99:1476-84. [Crossref] [PubMed]
- Lockney DT, Shub T, Hopkins B, et al. Spinal stereotactic body radiotherapy following intralesional curettage with separation surgery for initial or salvage chordoma treatment. Neurosurg Focus 2017;42. [Crossref] [PubMed]
- Yamaguchi T, Imada H, Iida S, et al. Notochordal Tumors: An Update on Molecular Pathology with Therapeutic Implications. Surg Pathol Clin 2017;10:637-56. [Crossref] [PubMed]
- Otani R, Mukasa A, Shin M, et al. Brachyury gene copy number gain and activation of the PI3K/Akt pathway: association with upregulation of oncogenic Brachyury expression in skull base chordoma. J Neurosurg 2018;128:1428-37. [PubMed]
- Bettegowda C, Yip S, Lo SL, et al. Spinal column chordoma: prognostic significance of clinical variables and T (brachyury) gene SNP rs2305089 for local recurrence and overall survival. Neuro Oncol 2017;19:405-13. [PubMed]
- Hindi N, Casali PG, Morosi C, et al. Imatinib in advanced chordoma: A retrospective case series analysis. Eur J Cancer 2015;51:2609-14. [Crossref] [PubMed]
- Hulen CA, Temple HT, Fox WP, et al. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am 2006;88:1532-9. [Crossref] [PubMed]
- Hanna SA, Aston WJ, Briggs TW, et al. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res 2008;466:2217-23. [Crossref] [PubMed]
- van Wulfften Palthe ODR, Janssen SJ, Wunder JS, et al. What questionnaires to use when measuring quality of life in sacral tumor patients: the updated sacral tumor survey. Spine J 2017;17:636-44. [Crossref] [PubMed]


