Surgery or not? A case of ventriculus terminalis in an adult patient
Introduction
The ventriculus terminalis (VT) is an ependyma-lined space located in the conus medullaris, which represents a normal developmental phase of the neural tube. It appears between the 43th and the 48th day of embryogenesis and regresses during the successive differentiation period. In some rare cases, it can persist in children and in adults, where it can be an occasional finding on radiological investigations or it can display insidious and non-specific symptoms due to progressive cerebrospinal fluid (CSF) accumulation and cystic enlargement of the cavity. The pathogenesis of VT persistency in adulthood and its cystic enlargement are still unclear and its management, whether conservative or surgical, is still an object of debate because of its rarity in the adult population.
Case presentation
A 52-year-old female patient with history of breast cancer, previously treated with chemo- and radiotherapy, presented with episodes of right leg clonus in the last 18 months, with hypoesthesia and weakness of the right thigh. Magnetic resonance imaging (MRI) of the spine showed an intramedullary cystic lesion at D10–D12 [length: 5.6 cm; volume: 6.6 cm3 (Figure 1A,B)], of which content was similar to CSF in both T1 and T2-weighted images without any post-contrast enhancement, and then reported as a terminal ventricle. No other pathological finding or spinal dysraphism at the lumbar and dorsal spine were evident. The patient was then addressed to conservative management and radiological follow-up.
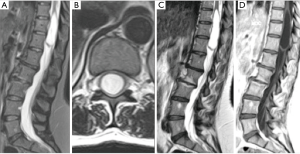
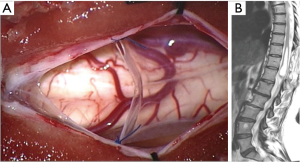
After one year, a new MRI showed an increase of the cystic lesion [length: 6.4 cm; volume: 7.9 cm3 (Figure 1C,D)], and the patient reported a worsening in both the clonus and the weakness at her right leg. Thus, we decided to perform a cyst—subdural marsupialization through D10–D12 laminectomy and median myelotomy (Figure 2A). During the surgical procedure the cystic content came out under pressure through the myelotomy walls and an amount was collected for cytological evaluation that confirmed the CSF nature of the sample. The immediate post-operative period was uneventful and the patient was discharged on day four. The 3-month follow-up MRI showed a marked reduction of the dimension of the intramedullary cavity [length: 5.9 cm; volume: 2.9 cm3 (Figure 2B)] and the patient referred an improvement of the weakness and no further episodes of clonus.
Discussion
The ventriculus terminalis (VT), or ‘fifth ventricle’ (1), is an ependyma-lined space located in the conus medullaris, containing CSF and communicating with the central canal of the anterior portion of the spinal canal. It represents a normal phase of the development of the neural tube, appearing between the 43th and the 48th day of embryogenesis, and disappearing during the regressive differentiation period (1). Nevertheless, a persistent VT can be documented as an occasional finding in children less than 5 years old (2). In adults, it is a very rare finding with only 57 documented cases reported to date (Table 1).
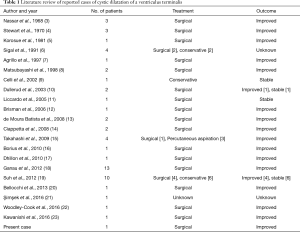
Full table
The etiopathogenesis is still unclear; the persistence of the VT can be caused by vascular disturbances, inflammatory diseases, compressions, ischemic necrosis of the spinal cord (3) or due to an isolation of the VT from the central canal resulting from its dysembriogenetic failed regression (11), with a progressive cystic dilatation of the cavity (6).
Up to now, this is the second case reported of a terminal ventricle that showed a progressive enlargement in the radiological follow-up (22).
Symptoms can be various, as some patients complain of non-specific symptoms (e.g.: low back pain, sciatica) and others exhibit focal neurological deficits, such as gait disturbances, paresis, muscular atrophy and sphincter dysfunction (13). The clinical presentation is also variable, ranging from an insidious onset (10,11) to a rapidly worsening form (6,8,10), even if only one case of an acute cauda syndrome has been reported (12).
The diagnosis was based on the MRI that showed a cystic dilatation at level of the cauda, with smooth walls and without internal septae, communicating with the spinal central canal. The cystic fluid has a similar signal to CSF on all sequences (hypointense on T1-weighted, isointense on proton density weighted and hyperintense on T2-weighted images) and does not show post-contrast enhancement. These characteristics are useful to discern it from other more common lesions at this level such as syringohydromyelia, intramedullary cyst or glial tumors. MRI is also useful in detecting other cranial or spinal conditions, such as spinal dysraphisms, Chiari malformation or tethered cord syndrome that are rare comorbidities in VT patients.
Because of the rarity of VT and the variability of the neurological condition in such patients, no standard treatment has been defined. Surgical management is represented by the fenestration of the cyst through laminectomy and a small midline myelotomy; after the drainage of the cavity the cyst is marsupialized to obtain a good CSF flow. This technique, similar to the one described by Nassar et al. (3), allows a complete recovery in 52% of cases and a partial recovery in 43% of cases (16). The placement of a shunt between the cyst walls and the subarachnoid space has been reported only in a few cases (4), albeit this procedure is still controversial because of a higher rate of complications and failure in the long-term follow-up. Recently, an alternative procedure of percutaneous aspiration of CSF during real-time MRI has been proposed with good results, but only in three patients (15).
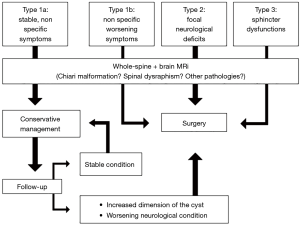
Because of these suboptimal outcomes, due to an empirical and subjective management, and considering that only patients with specific neurologic symptoms clearly related to VT showed a better outcome at the follow-up, de Moura B. et al. classified VT symptoms as three types and stated that surgical management should be reserved only in patients with neurological deficits or sphincter dysfunctions (Type 2–3), while conservative management, with a close neurological and radiological follow-up, should be preferred in patients with nonspecific complaints (Type 1) (13). Ganau et al. suggested a further subdivision of Type 1 symptoms into Type 1a, in which symptoms are non-specific and stable within a couple of months that can be managed conservatively, and Type 1b, with non-specific but worsening symptoms, requiring surgical management (18).
In our case the VT was originally an incidental finding because the first MRI was performed after the onset of clonus at her right leg and then treated surgically because of the dimensional increasing of the cyst and the worsening of the neurological condition. Before the surgical procedure the patient performed both brain and whole-spine MRI that did not show any other pathology (i.e., brain metastasis, Chiari malformation). The surgical drainage of the cyst content followed by subdural marsupialization was successful and the patient reported an improvement of her neurological condition, with evidence of a dimensional reduction of the cyst at the post-operative MRI.
To date, there is a lack of a standardized guideline for VT patients even if multiple authors have proposed a management chart subdividing the patients according to their symptoms and argue that only patients with neurological deficits or worsening condition should be treated surgically. We agree with this principle but we suggest performing a complete pre-operative radiological evaluation of both the brain and the spine to exclude the presence of other pathologies (i.e., brain/spinal tumor; Chiari malformation). Furthermore, clinical and radiological follow-up criteria should be included in the management chart for patients with non-specific symptoms (Type 1a), as shown in Figure 3.
Conclusions
A VT should not be considered as a steady, non-pathologic anatomical anomaly. In our case a VT showed a progressive enlargement with worsening of the neurological condition of the patient. Therefore, in both symptomatic and asymptomatic cases of fifth ventricle we suggest to perform a complete brain and spinal radiological investigation, in order to exclude other pathologies at these levels.
The surgical procedure of cyst fenestration through laminectomy and posterior median myelotomy is more advisable than a percutaneous aspiration of the cyst that has been described only for three patients and represents, in our opinion, a hazardous procedure due to a higher risk of damaging the rich perimedullary vascular network.
The rarity and unclear pathogenesis, together with the absence of a standard management and its unpredictable behaviour in adults had led to suboptimal results in VT patients, especially for those with non-specific neurological symptoms. We therefore stress the importance for further clinical studies and long term follow-up in operated patients to better define a univocal guideline for the management of this uncommon condition.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kernohan JW. The ventriculus terminalis: its growth and development. J Comp Neurol 1924;38:107-25. [Crossref]
- Coleman LT, Zimmerman RA, Rorke LB. Ventriculus terminalis of the conus medullaris: MR findings in children. AJNR Am J Neuroradiol 1995;16:1421-6. [PubMed]
- Nassar SI, Correll JW, Housepian EM. Intramedullary cystic lesions of the conus medullaris. J Neurol Neurosurg Psychiatry 1968;31:106-9. [Crossref] [PubMed]
- Stewart DH Jr, King RB, Lourie H. Surgical drainage of cyst of the conus medullaris. Report of three cases. J Neurosurg 1970;33:106-10. [Crossref] [PubMed]
- Korosue K, Shibasaki H, Kuroiwa Y, et al. Cyst of the conus medullaris manifesting amyotrophic lateral sclerosis syndrome. Folia Psychiatr Neurol Jpn 1981;35:507-10. [PubMed]
- Sigal R, Denys A, Halimi P, et al. Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatation. AJNR Am J Neuroradiol 1991;12:733-7. [PubMed]
- Agrillo U, Tirendi MN, Nardi PV. Symptomatic cystic dilatation of V ventricle: case report and review of the literature. Eur Spine J 1997;6:281-3. [Crossref] [PubMed]
- Matsubayashi R, Uchino A, Kato A, et al. Cystic dilatation of ventriculus terminalis in adults: MRI. Neuroradiology 1998;40:45-7. [Crossref] [PubMed]
- Celli P, D'Andrea G, Trillò G, et al. Cyst of the medullary conus: malformative persistence of terminal ventricle or compressive dilatation? Neurosurg Rev 2002;25:103-6. [Crossref] [PubMed]
- Dullerud R, Server A, Berg-Johnsen J. MR imaging of ventriculus terminalis of the conus medullaris. A report of two operated patients and a review of the literature. Acta Radiol 2003;44:444-6. [PubMed]
- Liccardo G, Ruggeri F, De Cerchio L, et al. Fifth ventricle: an unusual cystic lesion of the conus medullaris. Spinal Cord 2005;43:381-4. [Crossref] [PubMed]
- Brisman JL, Li M, Hamilton D, et al. Cystic dilation of the conus ventriculus terminalis presenting as an acute cauda equina syndrome relieved by decompression and cyst drainage: case report. Neurosurgery 2006;58:E585; discussion E585.
- de Moura Batista L, Acioly MA, Carvalho CH, et al. Cystic lesion of the ventriculus terminalis: proposal for a new clinical classification. J Neurosurg Spine 2008;8:163-8. [Crossref] [PubMed]
- Ciappetta P, D'urso PI, Luzzi S, et al. Cystic dilation of the ventriculus terminalis in adults. J Neurosurg Spine 2008;8:92-9. [Crossref] [PubMed]
- Takahashi S, Saruhashi Y, Odate S, et al. Percutaneous aspiration of spinal terminal ventricle cysts using real-time magnetic resonance imaging and navigation. Spine (Phila Pa 1976) 2009;34:629-34. [Crossref] [PubMed]
- Borius PY, Cintas P, Lagarrigue J. Ventriculus terminalis dilatation in adults: A case report and review of the literature. Neurochirurgie 2010;56:386-90. [Crossref] [PubMed]
- Dhillon RS, McKelvie PA, Wang YY, et al. Cystic lesion of the ventriculus terminalis in an adult. J Clin Neurosci 2010;17:1601-3. [Crossref] [PubMed]
- Ganau M, Talacchi A, Cecchi PC, et al. Cystic dilation of the ventriculus terminalis. J Neurosurg Spine 2012;17:86-92. [Crossref] [PubMed]
- Suh SH, Chung TS, Lee SK, et al. Ventriculus terminalis in adults: unusual magnetic resonance imaging features and review of the literature. Korean J Radiol 2012;13:557-63. [Crossref] [PubMed]
- Bellocchi S, Vidale S, Casiraghi P, et al. Multilobed cystic dilation of the ventriculus terminalis (CDVT). BMJ Case Rep 2013;2013. pii: bcr2013008654.
- Şimşek H, Zorlu E. Cystic lesion of the ventriculus terminalis accompanied by split cord malformation. Spine J 2016;16:e739. [Crossref] [PubMed]
- Woodley-Cook J, Konieczny M, Spears J. The Slowly Enlarging Ventriculus Terminalis. Pol J Radiol 2016;81:529-31. [Crossref] [PubMed]
- Kawanishi M, Tanaka H, Yokoyama K, et al. Cystic dilation of the ventriculus terminalis. J Neurosci Rural Pract 2016;7:581-3. [Crossref] [PubMed]


