One and two level posterior lumbar interbody fusion (PLIF) using an expandable, stand-alone, interbody fusion device: a VariLift® case series
Introduction
Degeneration of the spinal column generally begins in the second decade of life and continues to progress steadily, most commonly affecting the lower lumbar spine (1). Age-related lumbar degeneration of the motion segments can be associated with arthritis of the facet joints, decreased intervertebral disk height, imbalance, degenerative spondylolisthesis, and disk herniation or bulging (2). Facet joint degeneration can also cause spinal instability which can further damage the spine over time (2). These multifactorial conditions result in chronic low back pain that includes painful stiffness of the spine, radiculopathy, neurogenic claudication, and/or myelopathy. When spinal stenosis is present, narrowing of the spinal canal and the intervertebral foramen causes leg and buttocks pain, low back pain, paresthesia, and weakness.
When patients with degenerative spinal conditions fail to respond to conservative medical management, surgical interventions such as posterior lumbar interbody fusion (PLIF) with and without posterior instrumentation become an option. Intervertebral fusion devices with adjunctive pedicle screw fixation have been employed to ensure biomechanical stability due to nonunion development and increased instability associated with simple decompression methods without instrumentation (3,4). However, although widely used, traditional pedicle screw techniques have been associated with several complications such as screw loosening, breakage, and implant failure (5-7). To address many of these postoperative concerns, the VariLift® Interbody Fusion System was developed as a stand-alone solution to provide the benefits of the intervertebral fusion device without the requirement of supplemental pedicle screw fixation. Table 1 summarizes the advantages and disadvantages of stand-alone PLIF.

Full table
Descriptive patient outcome results for a case series of 25 patients who received the VariLift®-L are presented and discussed herein.
Methods
This retrospective case series was undertaken to evaluate the safety and clinical outcomes of 25 consecutive patients treated surgically in a single-physician practice between 2011 through 2013 for single or bi-level disc degeneration, instability, stenosis, disc rupture/herniation, spondylolisthesis, and/or facet disease. Preoperative symptoms included persistent low back pain and/or recurrent disc herniation refractory to at least 6 months of conservative care. All patients underwent a PLIF procedure with minimally-invasive implantation of the VariLift®-L interbody fusion system (Wenzel Spine, Austin, TX USA). In all cases, this stand-alone device was implanted without adjunctive posterior fixation such as pedicle screws and rods.
The design characteristics, indications for use, operative technique and clinical experience with the VariLift® device have been published previously (8,9). The VariLift®-L is a titanium, stand-alone, expandable interbody fusion device (Figure 1). Prior to surgical implantation, the device is cylindrical in shape with self-tapping threads, allowing for advancement into the intervertebral space without impaction. The device is then expanded in situ by advancing a sliding expansion plate to lock and secure the device in proper anatomical position. The bone graft chamber and fenestrations on all four sides of the implant allow for bone graft contact with the endplates to promote intervertebral bony fusion (9).
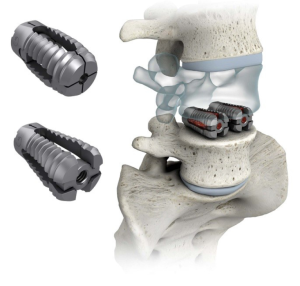
The surgical technique for a two-level PLIF with VariLift®-L is similar to the single-level PLIF procedure. In all cases, the operative technique consisted of discectomy and generous bilateral laminotomies with medial facetectomies preserving midline ligamentous structures. For both one- and two-level surgical procedures, the surgeon used milled autologous local bone admixed with Vitoss (Stryker®, Malvern, PA, USA) bone graft substitute and bone marrow aspirate to pack into the anterior disc space, in the midline between the cages and within the cages.
Clinical and radiologic assessments were performed preoperatively as well as at 6 and 12 months postoperatively. Prior to surgery and at each follow-up visit, patients were asked to describe the severity of their back pain as no pain, mild pain, moderate pain, severe pain or worst pain imaginable (10). Improvement in pain scores at 12 months compared to preoperative levels was assessed using McNemar’s test. Serial plain film radiographs were used to evaluate evidence of solid fusion or progression toward fusion. All radiographs were reviewed by the operating surgeon, and solid fusion was defined as presence of visible bone within the cage device, and absence of radiolucent halo effects around the implant and gross motion on dynamic films. All procedure-related postoperative complications and adverse events were tabulated.
Results
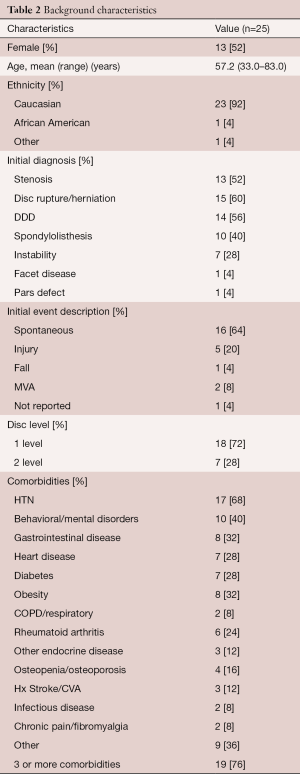
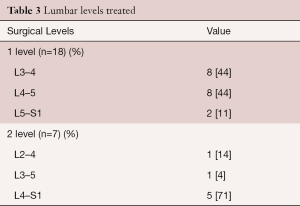
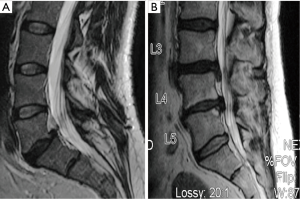
Background characteristics of this study group are provided in Table 2. The mean age was 57 years (range, 33–83 years) with an almost even gender distribution. Eighteen patients had a single level procedure and 7 patients had a two level procedure. All two level procedures were undertaken at contiguous levels (Table 3). The primary surgical indications were disc herniation, degenerative disc disease, stenosis and spondylolisthesis, often in combination. Figure 2 illustrates two typical cases of spinal degeneration on preoperative advanced imaging.
Full table
Full table
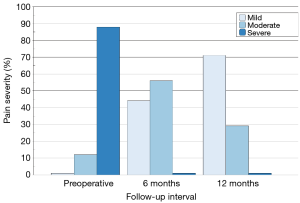
Preoperatively, 22 patients (88%) reported severe back pain with the remaining 3 patients (12%) reporting moderate back pain. All 7 patients treated at two levels described their back pain as severe prior to surgery. All patients returned for clinical follow-up assessments at 6 months and 21 patients returned at 12 months. Pain severity scores showed progressive improvement over time (Figure 3) with no patients reporting residual severe back pain at the 6- or 12-month follow-up visit. By 12 months, 71% of patients (15 of 21) described their back pain as mild. Overall, pain scores improved significantly from baseline to 12 months (P=0.0002). The degree of pain resolution following surgery was similar between patients having one and two level procedures.
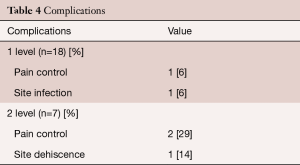
Postoperative complications are provided separately for patients with one and two level procedures in Table 4. Three patients complained of intractable back pain post-surgery, one patient suffered a wound infection identified as Staphylococcus aureus and Streptococcus anginosus that was treated with antibiotic therapy and resolved during the postoperative hospitalization period, and one patient had a surgical dehiscence that was successfully treated during inpatient hospitalization. Further examination of the 3 cases that did not experience pain resolution showed that the single level case had notable preoperative morbidities that included diabetes, obesity and a history of falls. One of the two level patients had undergone two prior lumbar surgeries and was actively undergoing pain management for fibromyalgia. The other two level patient also had a history of lumbar surgical interventions with prior removal of metallic instrumentation. No patients required subsequent implantation of adjunctive instrumentation or revision during the study follow-up period.
Full table
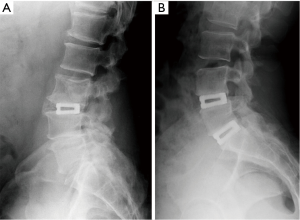
Eighteen patients (72%) provided radiographic follow-up at 6 months and 13 patients (52%) returned at 12 months for radiographic assessment. Solid fusion with absence of motion on dynamic films was noted in 92.3% (12 of 13) of patients at 12 months (Figure 4). Four patients with 6 month radiographs, who did not provide 12 month assessments, all showed progression toward fusion.
Discussion
This retrospective review of a small series of cases showed excellent clinical performance of a stand-alone, expandable interbody fusion device for the treatment of symptomatic disc degeneration and corroborates previous studies of stand-alone cages (11) as well as a recently published large case series of 470 patients treated with the VariLift®-L device (9). We employed a standard PLIF procedure which provides decompression of neural structures, axial loading of the anterior column, immediate restoration of disc height and foraminal patency, and compressive loading of the interbody bone graft.
The expandable design of the VariLift®-L converts the low-profile cylindrical implant to a wedge shaped implant when fully deployed to support preservation of lumbar lordosis. Previous reports have established the importance of restoring and maintaining lordosis with stand-alone cage technologies (12-14). Additionally, with the PLIF procedure, two bilateral devices are implanted (Figure 1), providing maximal endplate contact which has been shown to be preferable (15). When expanded, the large open fenestrations also allow for excellent radiographic visualization of the developing fusion construct (16).
We noted substantial symptom amelioration with the PLIF procedure utilizing the VariLift®-L device with 71% of patients reporting only mild residual pain 12 months after surgery. In those patients that returned for follow-up radiographic assessment, only one patient did not achieve solid fusion at 12 months resulting in a 92% fusion rate. This rate is consistent with previous reports of radiographic fusion success in degenerative disc disease (17). Finally, the PLIF procedure with the stand-alone VariLift®-L device was undertaken safely with few postoperative complications which were generally resolved during the inpatient hospitalization.
This case series review has several limitations foremost of which is the small sample size. Second, back pain severity was measured using a 5-part descriptor ranging from no pain to worst pain imaginable, which may not be as sensitive to changes in pain perception as the more typically-employed visual analog scale. Third, patients were not routinely evaluated for leg pain or back function using a standardized measure such as the Oswestry Disability Index. Inclusion of these measurements would have added clinical value to this report. Lastly, radiographic follow-up at 12 months was incomplete (52%) as many patients with satisfactory symptom amelioration failed to return for additional evaluation.
PLIF using the VariLift® device is an effective procedure that offers significant clinical success and high fusion rates. In this single-physician 25 patient case series, the VariLift® device used in a single or two-level PLIF demonstrated positive results with its stand-alone feature. The PLIF technique using VariLift®-L as a stand-alone device resulted in a 92% fusion rate, with minimal complications, patient-reported improved pain relief, and a quicker return to activities of daily living. The major advantage of using the VariLift® system is an improved surgical PLIF option for a stable interbody fusion without the need for supplemental pedicle screw fixation.
Acknowledgements
The authors thank Terry Meredith for graphical assistance. Special thanks to Beckinam Nowatzke for project management support.
Funding was provided by Wenzel Spine, Inc. (Austin, TX, USA) to support manuscript development.
Footnote
Conflicts of Interest: Dr. Block received support from Wenzel Spine to assist in manuscript development. Dr. Del Monaco is an employee of Wenzel Spine. Dr. Barrett-Tuck has no conflicts of interest to declare.
Ethical Statement: Local institutional review board (IRB) approval (BMH-IRB Number: 2) was obtained prior to the study and all patients provided written informed consent.
References
- Noshchenko A, Hoffecker L, Lindley EM, et al. Long-term Treatment Effects of Lumbar Arthrodeses in Degenerative Disk Disease: A Systematic Review With Meta-Analysis. J Spinal Disord Tech 2015;28:E493-521. [Crossref] [PubMed]
- Middleton K, Fish DE. Lumbar spondylosis: clinical presentation and treatment approaches. Curr Rev Musculoskelet Med 2009;2:94-104. [Crossref] [PubMed]
- Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009;2:118-26. [Crossref] [PubMed]
- France JC, Yaszemski MJ, Lauerman WC, et al. A randomized prospective study of posterolateral lumbar fusion. Outcomes with and without pedicle screw instrumentation. Spine (Phila Pa 1976) 1999;24:553-60. [Crossref] [PubMed]
- Babu R, Park JG, Mehta AI, et al. Comparison of superior-level facet joint violations during open and percutaneous pedicle screw placement. Neurosurgery 2012;71:962-70. [Crossref] [PubMed]
- Galbusera F, Volkheimer D, Reitmaier S, et al. Pedicle screw loosening: a clinically relevant complication? Eur Spine J 2015;24:1005-16. [Crossref] [PubMed]
- Patel RD, Graziano GP, Vanderhave KL, et al. Facet violation with the placement of percutaneous pedicle screws. Spine (Phila Pa 1976) 2011;36:E1749-52. [Crossref] [PubMed]
- Emstad E, Del Monaco DC, Fielding LC, et al. The VariLift(®) Interbody Fusion System: expandable, standalone interbody fusion. Med Devices (Auckl) 2015;8:219-30. [PubMed]
- Neely WF, Fichtel F, Del Monaco DC, et al. Treatment of Symptomatic Lumbar Disc Degeneration with the VariLift-L Interbody Fusion System: Retrospective Review of 470 Cases. Int J Spine Surg 2016;10:15. [Crossref] [PubMed]
- Gracely RH, Kwilosz DM. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment. Pain 1988;35:279-88. [Crossref] [PubMed]
- Lequin MB, Verbaan D, Bouma GJ. Posterior lumbar interbody fusion with stand-alone Trabecular Metal cages for repeatedly recurrent lumbar disc herniation and back pain. J Neurosurg Spine 2014;20:617-22. [Crossref] [PubMed]
- Barrey C, Darnis A. Current strategies for the restoration of adequate lordosis during lumbar fusion. World J Orthop 2015;6:117-26. [Crossref] [PubMed]
- Gödde S, Fritsch E, Dienst M, et al. Influence of cage geometry on sagittal alignment in instrumented posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2003;28:1693-9. [Crossref] [PubMed]
- Kim JT, Shin MH, Lee HJ, et al. Restoration of lumbopelvic sagittal alignment and its maintenance following transforaminal lumbar interbody fusion (TLIF): comparison between straight type versus curvilinear type cage. Eur Spine J 2015;24:2588-96. [Crossref] [PubMed]
- Zhang JD, Poffyn B, Sys G, et al. Are stand-alone cages sufficient for anterior lumbar interbody fusion? Orthop Surg 2012;4:11-4. [Crossref] [PubMed]
- Schiffman M, Brau SA, Henderson R, et al. Bilateral implantation of low-profile interbody fusion cages: subsidence, lordosis, and fusion analysis. Spine J 2003;3:377-87. [Crossref] [PubMed]
- Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;32:1155-62; discussion 1163. [Crossref] [PubMed]
Contributions: (I) Conception and design: R Barrett-Tuck, D Del Monaco; (II) Administrative support: Terry Meredith; (III) Provision of study materials or patients: R Barrett-Tuck; (IV) Collection and assembly of data: D Del Monaco; (V) Data analysis and interpretation: D Del Monaco, JE Block; (VI) Manuscript writing: All authors; (VII): Final approval of manuscript: All authors.


