Bone bridge formation across the neuroforamen 14 years after instrumented fusion for isthmic spondylolisthesis—a case report
Introduction
In situ, instrumented, posterolateral fusion is an accepted surgical management of isthmic spondylolisthesis. For many years, this treatment has yielded good results, with low complication rates (1,2). Recurrence of radiculopathy due to heterotopic bone formation across the neuroforamen has not been previously reported in this group of patients. This could be due to a lack of long-term follow-up studies evaluating this complication.
The authors believe that surgical factors play a substantial role in determining the occurrence of this complication. In this report, we describe the first case of the aforementioned complication presenting 14 years post-operatively, and discuss surgical strategies that may aid in its prevention.
Case presentation
In 2000, a 70-year-old lady presented with a 2-year history of mechanical low back pain which had worsened over the previous 3 months, particularly during extension of her back. This was associated with neurogenic claudication upon walking distances approximating 500 meters, which she described as bilateral lower limb pain radiating down the posterior aspects of her thighs and lateral aspects of her legs. This pain was relieved by sitting and bending forwards. She also complained of paresthesia in the same distribution. Her Oswestry Disability Index (ODI) score was 56.
Physical examination revealed a step deformity of the lower back and associated paravertebral muscle spasms. The patient had reduced lumbar range of motion, with lumbar excursion limited to 3 centimeters. Power of her lower limbs was intact, although ankle jerks were hyporeflexic bilaterally. There was no clonus in her lower limbs, and sensation was preserved bilaterally. Straight leg raise test was negative in both lower limbs.
During this initial presentation, anterior-posterior and lateral lumbar spine X-rays showed a grade 2 isthmic spondylolisthesis at L5/S1.The patient also demonstrated instability in the flexion and extension X-ray views of her lumbar spine. She had underwent non-surgical therapy including back care advice, lifestyle modifications and physiotherapy for a total of 3 months with no improvement in her symptoms.
A subsequent magnetic resonance imaging (MRI) of the lumbar spine confirmed the diagnosis of isthmic spondylolisthesis and showed bilateral L5/S1 lateral recess stenosis, compressing on the S1 nerve roots.
In view of these persistent symptoms, the patient underwent decompression laminectomy of L5 and in situ posterolateral fusion, with instrumentation at the L5/S1 level using local morcelized bone graft on decorticated transverse processes of L5 and S1 alae. Decortication was performed using a high-speed burr. No graft extenders or bone morphogenetic protein (BMP) was used. Both L5 exiting nerve roots and S1 traversing nerve roots were assessed to be free from compression. The operation was uneventful and post-operatively, her lower limb symptoms resolved completely. Her ODI score was 32. She was on annual follow-up for the next 14 years and remained asymptomatic during this period.
Fourteen years post-operatively, the patient presented with a 6-month history of progressive onset, radicular pain in the distribution of her left buttock, posterolateral thigh and lateral leg. The pain was constant and was exacerbated by physical activities such as walking continuously for 5 minutes or prolonged standing for 10 minutes. There was no right leg pain. The patient did not have any lower limb weakness or numbness, as well as changes in bladder and bowel habit.
On examination, the patient had a healed midline scar at her lower back. Lumbar range of motion was reduced, with an excursion of 2 cm. Neurological examination of the lower limbs revealed weakness (Grade 4 power) in left big toe dorsiflexion, but intact sensation in all dermatomes. Ankle reflexes remained hyporeflexic bilaterally. The straight leg raise test was negative at 80 degrees in both lower limbs.
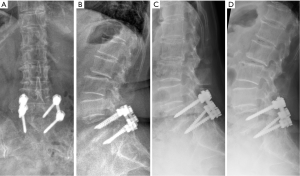
Anterior-posterior (Figure 1A) and lateral (Figure 1B) X-rays performed showed interval changes with worsening degeneration at the L1/2, L2/3 and L4/5 levels. The vacuum sign was seen at L1/2 and L2/3. Retrolisthesis, associated with dynamic instability during flexion (Figure 1C) and extension stress views (Figure 1D) was also observed at the L2/3level. There was no movement seen at the L5/S1 level which indicated successful fusion.
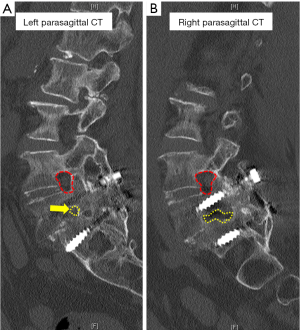
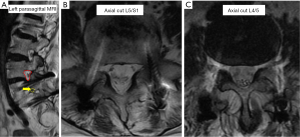
Computed tomography (CT) scans (Figure 2) showed complete L5/S1 posterolateral fusion and an acceptable implant position without evidence of loosening. No further degenerative changes were found at the fused level. However, a bone bridge that spanned from the previous lysis defect at the left pars interarticularis to the superior endplate of the S1 vertebra had formed across the left L5/S1 neuroforamen (Figure 2A). This was absent on the right side (Figure 2B). This bone bridge bisected the neuroforamen, resulting in stenosis and possible compression of the exiting left L5 nerve root as seen on the MRI (Figure 3A,B). There was no compression of the traversing left L5 nerve root (Figure 3C), indicating that the patient’s radicular symptoms were likely caused by compression of the exiting L5 nerve root at the left L5/S1 level.
In view of the patient’s concordant symptoms, a diagnostic nerve root block was offered. However, the patient opted for conservative treatment with oral analgesics and physiotherapy which did not relieve her symptoms. She continues to be symptomatic after 1-year of follow-up with ODI score of 60.
Discussion
This study reports on a patient with a primary condition of grade 2 L5S1 isthmic spondylolisthesis that underwent successful L5 decompression laminectomy and L5/S1 instrumented posterolateral fusion. The patient was symptom free for 13 years before developing left L5 neuroforamen stenosis secondary to bone bridge formation. Despite ongoing symptoms, patient opted for conservative treatment.
Although many possible hypotheses on the etiology of spondylolysis have been described [including hereditary, dysplastic, mechanical and traumatic factors (3-5)], it is widely accepted that mechanical stress across the pars interarticularis (6-9) is the eventual triggering factor which results in formation of the spondylolysis defect. Wiltse proposed that most cases of spondylolysis occur due to repetitive stress at said portion of the vertebra, leading to fatigue failure (7). However, unlike other fatigue fractures that usually heal with time, these defects often persist (7). From a biomechanical perspective, this may be attributed to the constant separation of the pars interarticularis secondary to distracting forces acting across the lysis defect, in addition to the poor blood supply to the region. This instability causes patients to experience low back pain upon extension of the lumbar spine (7,10-12), and may eventually lead to intervertebral disc failure and spondylolisthesis (13). Surgical management of this condition in the form of instrumentation and fusion to achieve biomechanical stability have generally yielded good results (14,15).
The unique nature of this case lies in the extremely rare complication of a bone bridge forming across the neuroforamen, between the exposed end plate and the lysis defect after in-situ, instrumented, stabilization surgery. This could either stem from lack of long-term patient follow-ups or a myriad of surgically-related reasons. In elucidating the possible etiologies for this phenomenon, we considered the biological and mechanical factors that favored bone formation and union. The biological prerequisites include the presence of local osteogenic, osteoinductive and osteoconductive factors, as well as the presence of adequate blood supply (16,17). The mechanical determinants include permissible strain at the bony surfaces as described by Perren (18,19), which is dependent on the stability across these surfaces conferred by the strength of fixation.
The formation of bone bridge across the neuroforamen, between a healed pars interarticularis and the superior endplate of the caudad vertebra has only been described once before, in the context of a patient who had an interbody fusion performed (20). In that case, although no BMP was used, the end plate preparation and introduction of autologous bone graft across the spinal canal could have led to increased local concentration of osteogenic and osteoinductive factors. This could have triggered bone growth and led to the formation of the bone bridge over time. This case was unique in that bone bridge formation occurred when only posterolateral fusion was performed, without the preparation of vertebral end plates. Furthermore, no bone graft or BMP was introduced across the neuroforamen or central spinal canal. This suggests that patients with isthmic spondylolisthesis could inherently have sufficient local osteogenic and osteoinductive factors for bone formation, although this may be prevented by the lack of stability. After instrumentation, the stable local environment could inadvertently have allowed osteogenic and osteoinductive factors to form bone across the neuroforamen.
While it is desirable to create a permissive environment for bone formation in spine fusion surgery, this case demonstrated that heterotopic ossification within the spinal canal could also potentially result in long-term complications such as exit nerve root compression at the neuroforamen. Despite the lack of objective evidence to suggest that the new left L5 radicular pain could be a result of neuroforaminal stenosis, concordant intraoperative findings at the index operation, patient’s current symptoms and findings on the MRI/CT scans strongly suggest this to be the cause. Hence, there is strong cause for us to highlight this case with the intention that further studies look at similar patients’ long-term outcomes. In retrospect, the authors propose two precautions to be instituted at the time of initial surgery to prevent this potential complication.
First, we suggest stronger consideration for the reduction of the spondylolisthesis. Reduction of spondylolisthesis has not been deemed compulsory in the treatment of isthmic spondylolisthesis (21). It is only strongly recommended when there is a need to increase surface contact between vertebral end plates to facilitate interbody fusion (22,23) or to restore lumbosacral parameters and sagittal spinal balance (24,25). Mac-Thiong et al., have classified lumbosacral spondylolisthesis based on the grade of slip, degree of dysplasia and sagittal spinopelvic balance, and provided corresponding recommendations regarding surgical management (26). Reduction of the slip has been acknowledged as a controversial issue due to the lack of conclusive data and the authors only recommended it for patients with high grade slips for the above-mentioned reasons. Theoretically, bone formation between the pars defect and the caudad vertebra’s superior end plate is less likely if the spondylolisthesis is reduced, because the distance across the neuroforamen between the two exposed bone surfaces is increased. Although interfragmentary gaps greater than 2 mm have been previously reported to inhibit bone healing (27), based on the CT scan, bone bridge formation in our patient occurred over an estimated 3–4 mm. Therefore, it may be prudent to achieve as much reduction as safely possible until more evidence for a “safe distance” is available.
Second, we suggest resection of pars interarticularis and its associated fibro-granulation tissue. This technique is used to directly decompress neural elements compromised by the spondylolisthesis (28,29). However, this is not routinely practiced and some surgeons prefer indirect decompression via interbody distraction with spacers, as well as reduction of the spondylolisthesis (30-32). We believe that resection of the pars interarticularis and its associated fibrogranulation tissue may eliminate the possibility of bone bridge formation from the lysis defect, across the neuroforamen, as in this case. Therefore, this option may be considered especially in cases where reduction of spondylolisthesis is deemed unnecessary.
Conclusions
In conclusion, our patient who underwent in situ posterolateral fusion and instrumentation for a condition of L5/S1 isthmic spondylolisthesis, developed stenosis at the left L5/S1 neuroforamen secondary to a bone bridge. Although there may be multiple contributing factors to this complication, this case raises certain issues that should be further explored in future studies. Theoretically, this may have been prevented if resection of both pars interarticularis and reduction of the spondylolisthesis had been performed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
References
- Madan S, Boeree NR. Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine (Phila Pa 1976) 2002;27:1536-42. [Crossref] [PubMed]
- Lee GW, Lee SM, Ahn MW, et al. Comparison of posterolateral lumbar fusion and posterior lumbar interbody fusion for patients younger than 60 years with isthmic spondylolisthesis. Spine (Phila Pa 1976) 2014;39:E1475-80. [Crossref] [PubMed]
- Nazarian S. Spondylolysis and spondylolytic spondylolisthesis. A review of current concepts on pathogenesis, natural history, clinical symptoms, imaging, and therapeutic management. Eur Spine J 1992;1:62-83. [Crossref] [PubMed]
- Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am 1984;66:699-707. [Crossref] [PubMed]
- Tsirikos AI, Garrido EG. Spondylolysis and spondylolisthesis in children and adolescents. J Bone Joint Surg Br 2010;92:751-9. [Crossref] [PubMed]
- Letts M, Smallman T, Afanasiev R, et al. Fracture of the pars interarticularis in adolescent athletes: a clinical-biomechanical analysis. J Pediatr Orthop 1986;6:40-6. [Crossref] [PubMed]
- Wiltse LL, Widell EH Jr, Jackson DW. Fatigue fracture: the basic lesion is inthmic spondylolisthesis. J Bone Joint Surg Am 1975;57:17-22. [Crossref] [PubMed]
- Farfan HF, Osteria V, Lamy C. The mechanical etiology of spondylolysis and spondylolisthesis. Clin Orthop Relat Res 1976.40-55. [PubMed]
- O'Neill DB, Micheli LJ. Postoperative radiographic evidence for fatigue fracture as the etiology in spondylolysis. Spine (Phila Pa 1976) 1989;14:1342-55. [Crossref] [PubMed]
- Soler T, Calderón C. The prevalence of spondylolysis in the Spanish elite athlete. Am J Sports Med 2000;28:57-62. [PubMed]
- Anderson SJ. Assessment and management of the pediatric and adolescent patient with low back pain. Phys Med Rehabil Clin N Am 1991;2:157-85. [Crossref]
- Stinson JT. Spondylolysis and spondylolisthesis in the athlete. Clin Sports Med 1993;12:517-28. [PubMed]
- Louis R. Bases anatomo-pathologiques. (Symposium, Le spondylolisthesislombo-sacr6) Rev Chit Orthop 1971;57:99-105.
- Snyder LA, Shufflebarger H, O'Brien MF, et al. Spondylolysis outcomes in adolescents after direct screw repair of the pars interarticularis. J Neurosurg Spine 2014;21:329-33. [Crossref] [PubMed]
- Drazin D, Shirzadi A, Jeswani S, et al. Direct surgical repair of spondylolysis in athletes: indications, techniques, and outcomes. Neurosurg Focus 2011;31:E9. [Crossref] [PubMed]
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 1998.Suppl:S7-21. [Crossref] [PubMed]
- Einhorn TA. The science of fracture healing. J Orthop Trauma 2005;19:S4-6. [Crossref] [PubMed]
- Perren SM. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop Relat Res 1979.175-96. [PubMed]
- Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing--an update. Injury 2007;38 Suppl 1:S3-10. [Crossref] [PubMed]
- Wagner SC, Kang DG, Helgeson MD. Heterotopic ossification after transforaminal lumbar interbody fusion without bone morphogenetic protein use. Spine J 2014;14:2783-4. [Crossref] [PubMed]
- Longo UG, Loppini M, Romeo G, et al. Evidence-based surgical management of spondylolisthesis: reduction or arthrodesis in situ. J Bone Joint Surg Am 2014;96:53-8. [Crossref] [PubMed]
- Hakało J, Wroński J. The role of reduction in operative treatment of spondylolytic spondylolisthesis. Neurol Neurochir Pol 2008;42:345-52. [PubMed]
- Spruit M, Pavlov PW, Leitao J, et al. Posterior reduction and anterior lumbar interbody fusion in symptomatic low-grade adult isthmic spondylolisthesis: short-term radiological and functional outcome. Eur Spine J 2002;11:428-33. [Crossref] [PubMed]
- Hresko MT, Labelle H, Roussouly P, et al. Classification of high-grade spondylolistheses based on pelvic version and spine balance: possible rationale for reduction. Spine (Phila Pa 1976) 2007;32:2208-13. [Crossref] [PubMed]
- Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024-9. [Crossref] [PubMed]
- Mac-Thiong JM, Labelle H. A proposal for a surgical classification of pediatric lumbosacral spondylolisthesis based on current literature. Eur Spine J 2006;15:1425-35. [Crossref] [PubMed]
- Claes L, Augat P, Suger G, et al. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res 1997;15:577-84. [Crossref] [PubMed]
- Shiraishi T, Crock HV. Excision of laminal pseudoarthroses in spondylolytic spondylolisthesis. A review of 13 cases. Eur Spine J 1995;4:52-5. [Crossref] [PubMed]
- Gill GG, Manning JG, White HL, et al. Surgical treatment of spondylolisthesis without spine fusion; excision of the loose lamina with decompression of the nerve roots. J Bone Joint Surg Am 1955;37-A:493-520. [Crossref] [PubMed]
- Ani N, Keppler L, Biscup RS, et al. Reduction of high-grade slips (grades III-V) with VSP instrumentation. Report of a series of 41 cases. Spine (Phila Pa 1976) 1991;16:S302-10. [Crossref] [PubMed]
- DeWald CJ, Vartabedian JE, Rodts MF, et al. Evaluation and management of high-grade spondylolisthesis in adults. Spine (Phila Pa 1976) 2005;30:S49-59. [Crossref] [PubMed]
- Tallarico RA, Lavelle WF, J, Bianco A, et al. Positional effects of transforaminal interbody spacer placement at the L5-S1 intervertebral disc space: a biomechanical study. Spine J 2014;14:3018-24. [Crossref] [PubMed]


