Surfer’s myelopathy: a rare presentation in a non-surfing setting and review of the literature
Introduction
First defined by Thompson et al. [2004], surfer’s myelopathy (SM) is a rare diagnosis of acute non-traumatic spinal cord infarction most commonly found in first time surfing patients (1). Postulated to only be driven by the excessive forces applied during surfing, from both manoeuvring and the surrounding waves, the underlying pathophysiology is driven by hyperextension of the spine leading to vascular damage and consequent ischaemia to distal spinal cord segments. The hyperextension itself is believed to increase tension on both the spinal cord and surrounding vasculature, causing possible avulsion of perforating vessels and secondary vasospasm leading to transient ischaemia. This theory has recently been challenged in a recent review by Freedman et al. [2016] suggesting other mechanisms including inferior vena caval compression while surfers lie prone on the board (prolonged valsalva manoeuvre), or embolization in the central/sulcal arteries secondary to spinal disk damage (2).
Regardless of etiology, evidence of cord infarction is demonstrable through magnetic resonance imaging (MRI) following presentation, with a classical T2 hyperintensity visible on a longitudinal view. Deterioration is rapid (within hours), with presentation variable including symptoms of back pain, paraplegia, hyperalgesia and non-specific neurological symptoms (3). Similar to presentation, rates of improvement are equally as variable, ranging from no improvement to complete recovery. Lastly, It is important to recognise that the majority of patients are young and generally have no existing spinal or vascular pathology. Hence delayed (>24 hours) presentation may have significant impact on overall outcomes.
Although there have been 64 cases published since 2004, standardisation in the management algorithm of these patients is yet to be achieved (1,2,4-18). Several interventions have been proposed, including early steroid therapy and anticoagulation, though the rarity of cases has largely prevented quality research on the matter. Freedman et al. [2016] have hypothesised a possible association between existing hypoperfusion and the development of SM, as demonstrated in other causes of spinal infarction, but failed to back up their claims with angiographic data (n=1) (2). No other studies have evaluated the underlying vascular changes with angiography.
The purpose of the present report is (1) to present a possible case of SM involving a non-surfing mechanism and (2) to establish a succinct management algorithm in patients presenting with possible SM.
Case presentation
A 51-year-old male demolition worker who presented after commencing work with bilateral lower limb paraesthesia and numbness 2 days prior. He reported acute deterioration of the pain while conducting usual lifting procedures within half an hour, eventually progressing to a sensory loss inferiorly from the pelvis and overflow incontinence within two hours. There were no other associated symptoms. He had lost fifteen kilograms of weight over the previous 12 months, and reported a past mechanical fall that he had since recovered from. Risk factor screening revealed a 12-pack year smoking history and mild alcohol consumption.
On examination the patient was afebrile with unremarkable vital signs. The neck was soft with no rigidity and he had no spinal deformity. Lower limbs examination revealed symmetrical power changes. Hip extension and flexion was 4/5, knee extension and flexion was 4+/5, ankle dorsiflexion was 2/5 and ankle plantar flexion was 3/5. Tone was increased bilaterally with no presence of clonus. He had a sensory level (all sensation) to L4 with decreased perianal and perineal sensation. Anal tone was lax. The remainder of the neurological examination was unremarkable. These findings were consistent with a incomplete spinal cord injury (ASIA C) with impaired bowel function.
T2 weighted MRI (Figure 1) demonstrated expansion and abnormal hyperintensity involving the conus medullaris of the spinal cord level with T12 and L1. Diffusion weighted and gadolinium contrast MRI (Figure 1) revealed diffusion restriction signals corresponding to the previous hyperintense T2 signal detected. This was consistent with a recent infarct at the level. No abnormal contrast enhancement was seen and there was no suggestion of disk herniation. CT angiography of the abdomen and pelvis showed normal vessels with normal flow. No aneurysms or thrombi were seen. Catheter angiography was not performed due to concern of further spinal cord insult.
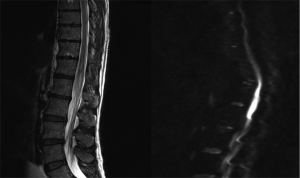
Other investigations performed included a chest X-ray, CT-brain, and CT-abdomen/pelvis. Blood tests included an FBC, EUC, CMP, LFT, coagulation studies, CRP, ESR and immunology tests were unremarkable and was negative for vasculopathy.
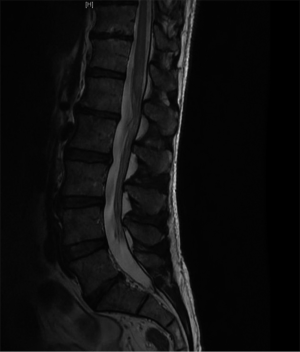
The patient was commenced on intravenous dexamethasone with almost immediate improvement in sensation, supplemented with intravenous fluid therapy to regulate systolic blood pressure and urinary catheterisation due to retention. He was discharged six days later mobilising independently with repeat MRI (Figure 2) demonstrating no proximal infarct extension. At discharge he was actively weaning off the dexamethasone and reported ongoing saddle and perineal anaesthesia.
Discussion
Although the overwhelming majority of literature surrounding SM is on young surfing patients, our report is not the first to demonstrate consistent features with a non-surfing etiology (7). Wadia et al’s [2015] case report involved a 7-year-old female cheerleader, with a hyperextension movement elicited upon history. Other risk factors initially proposed by Thompson et al. include: thin body habitus with underdeveloped musculature, dehydration and long distance travel (1). Dehydration itself may contribute to a hyper-coagulable state however its role in the aetiology has not been previously assessed in any available studies. Regardless, in our present case there was no history of either set of risk factors or an apparent history of a hyperextension motion.
This raises the issue that while the presence of surfing and acute hyperextension may have previously been predictors of SM, the diagnosis should be considered in all cases involving strenuous activity to the spine. This however is not consistent with the etiological hypothesis surrounding SM made by Freedman et al. [2016] and directly contradicts the previous beliefs of occurrence in only young non-experienced surfers (2). The most recent proposed mechanism by Freedman et al. suggest that the underlying ischaemia is more likely to be driven by IVC compression or embolization within the spinal arteries themselves, secondary to prolonged hyperextension. In the setting of surfing this relates to the prone position on the board. This factor was not formerly assessed in our case but has been supported by other studies (6,15). Naturally, this explains why the lower spinal cord and conus medullaris may be vulnerable to arterial insufficiency as evident in the majority of published literature. Lastly, due to this mechanism and the acute nature of myelopathy, the presence of disc herniation should be considered as a relevant differential.
Other suggested pathophysiological mechanisms include vascular avulsion of perforating vessels, vasospasm of the artery of Adamkiewicz, and fibrocartilaginous disc embolisation. Due to the possible presence of vascular injury, the routine use of angiography during the diagnostic phase of care was implemented in a single study (n=1) however failed to locate any pathology. While the use of angiography does allow visualisation of the any emboli present, with the potential of follow up angiography available as a comparison following any vascular intervention, we do agree that the test maybe of some value but there is a concern for angiography related complications. In our present case, although it was not determined, the senior authors speculate the underlying cause to be related to spinal cord hypoperfusion.
The largest studies by Nakamoto (n=23) and Chang (n=19) implement routine MRI as the most important diagnostic tool, with the latter showing increased diagnostic accuracy (6/10 patients) associated with the addition of Diffusion weighted imaging (DWI)—consistent with the ischaemic aetiology (4,8). MRI-DWI itself is highly sensitive to detecting acute cellular injury due to the changes in water diffusion present secondary to the ischaemia (11). In both studies in the inclusion of gadolinium contrast did not facilitate diagnosis, but was justified as a means of excluding differentials. Hence the classical appearance is a normal T1 MRI with a longitudinal hyperintense lesions extending distally from the mid-thoracic region to the conus on the T2-MRI. The relative size of the lesion is expected to increase within the first 24h of injury. In Nakamoto et al.’s study a lumbar puncture was performed in 12/19 cases, with extremely variable results. We believe this additional procedure to be of limited use in this setting, and believe it should not be performed unless there are other clearer indications.
Finally, there is limited data on interventions for SM, which obviates the significant inconsistency in patient outcomes reported. As shown in Table 1, adapted from a recent review by Freedman et al. [2016], the routine use of steroids has been of benefit in a mere 55% of cases (n=22) (2). Early steroid therapy has been efficacious in the setting of acute cord injuries including transverse myelitis (key differentials), hence, as SM patients are generally at minimal risk of adverse effects due to the low duration of therapy, is appropriate to initiate (2,10,19). In our case the patient recovered rapidly following his admission, and aside from fluid management he did not receive any further therapy aside from the steroids.
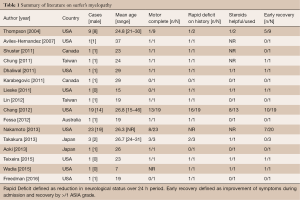
Full table
Finally with shifting thought towards the vascular changes underlying the onset of disease, it has been suggested that there may be a role for routine tPA administration (2), however there is limited available evidence to date to assess the relative benefits and risks of this approach.
In summary our recommendations in the setting of possible SM would be to first establish diagnosis through history, clinical examination and imaging (MRI, MRI-DWI). The patient should be admitted as early as possible (ideally <24 h) and commenced on steroid therapy (controversial) with aggressive monitoring of blood pressure and constant patient outcomes reviewing taking place over the next 72 hours. The use of rehabilitation services may be useful if available.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this mauscript and any accompanying images.
References
- Thompson TP, Pearce J, Chang G, et al. Surfer's myelopathy. Spine (Phila Pa 1976) 2004;29:E353-6. [Crossref] [PubMed]
- Freedman BA, Malone DG, Rasmussen PA, et al. Surfer's Myelopathy: A Rare Form of Spinal Cord Infarction in Novice Surfers: A Systematic Review. Neurosurgery 2016;78:602-11. [Crossref] [PubMed]
- Conidi F. Some unusual sports-related neurologic conditions. Continuum (Minneap Minn) 2014;20:1645-56. [Crossref] [PubMed]
- Chang CW, Donovan DJ, Liem LK, et al. Surfers' myelopathy: a case series of 19 novice surfers with nontraumatic myelopathy. Neurology 2012;79:2171-6. [Crossref] [PubMed]
- Teixeira S, Moser F, Kotton RH. Imaging features and differentials in surfer's myelopathy: a case report. Emerg Radiol 2016;23:89-92. [Crossref] [PubMed]
- Fessa CK, Lee BS. An Australian case of surfer's myelopathy. Clin J Sport Med 2012;22:281-3. [Crossref] [PubMed]
- Wadia S, Padmanabhan P, Moeller K, et al. Pediatric Surfer's Myelopathy. J Emerg Med 2015;49:e143-5. [Crossref] [PubMed]
- Nakamoto BK, Siu AM, Hashiba KA, et al. Surfer's myelopathy: a radiologic study of 23 cases. AJNR Am J Neuroradiol 2013;34:2393-8. [Crossref] [PubMed]
- Takakura T, Yokoyama O, Sakuma F, et al. Complete paraplegia resulting from surfer's myelopathy. Am J Phys Med Rehabil 2013;92:833-7. [Crossref] [PubMed]
- Karabegovic A, Strachan-Jackman S, Carr D. Surfer's myelopathy: case report and review. CJEM 2011;13:357-60. [Crossref] [PubMed]
- Lieske J, Cameron B, Drinkwine B, et al. Surfer's myelopathy-demonstrated by diffusion-weighted magnetic resonance imaging: a case report and literature review. J Comput Assist Tomogr 2011;35:492-4. [Crossref] [PubMed]
- Lin CY, Fu JH, Li SC, et al. Surfer's myelopathy. QJM 2012;105:373-4. [Crossref] [PubMed]
- Chung HY, Sun SF, Wang JL, et al. Non-traumatic anterior spinal cord infarction in a novice surfer: a case report. J Neurol Sci 2011;302:118-20. [Crossref] [PubMed]
- Shuster A, Franchetto A. Surfer's myelopathy--an unusual cause of acute spinal cord ischemia: a case report and review of the literature. Emerg Radiol 2011;18:57-60. [Crossref] [PubMed]
- Dhaliwal PP, Cenic A, Eesa M, et al. An unusual case of myelopathy: surfer's myelopathy. Can J Neurol Sci 2011;38:354-6. [Crossref] [PubMed]
- Aoki M, Moriizumi S, Toki M, et al. Rehabilitation and long-term course of nontraumatic myelopathy associated with surfing. Am J Phys Med Rehabil 2013;92:828-32. [Crossref] [PubMed]
- Avilés-Hernández I, García-Zozaya I, DeVillasante JM. Nontraumatic myelopathy associated with surfing. J Spinal Cord Med 2007;30:288-93. [Crossref] [PubMed]
- Dimmick S, Brazier D, Wilson P, et al. Injuries of the spine sustained whilst surfboard riding. Emerg Radiol 2013;20:25-31. [Crossref] [PubMed]
- Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev 2012;1:CD001046. [PubMed]


