
Restoring spinopelvic harmony with lateral lumbar interbody fusion: is it a realistic goal?
Introduction
Spinopelvic harmony is undeniably critical for the efficient function of the musculoskeletal system (1-6). A widely accepted radiological target to achieve spinopelvic harmony via corrective lumbar surgery is a lumbar lordosis (LL) within 10 degrees of the pelvic incidence (PI) (4-7). Values lying outside of this range, known as the PI-LL mismatch, have been shown to increase shear forces within the lumbar spine (8), leading to increased fatigue and muscular demand (1,2,7,9). Furthermore, a strong literature base exists confirming the role of PI-LL mismatch in adverse quality of life outcome measures (5,7,9-11), as well as placing patients at increased risk of adjacent segment degeneration (12-14).
Traditionally, sagittal deformity correction has been performed via open approaches, including shortening of the posterior column with Smith-Petersen-type or pedicle subtraction osteotomies (PSOs) (15-18). Although effective, such procedures carry the risk of increased blood loss, operative times, and neurological complications, all of which confer significant morbidity (15-19). Since its introduction in 2006 (20), the lateral lumbar interbody fusion (LLIF) technique has gained interest with respect to its application in a myriad of spinal pathologies (21-24). Multiple centres have adapted its use as an adjunct in deformity surgery (25-29). There is a wealth of data confirming its ability to provide indirect foraminal decompression and improved patient outcomes (27,30-37). However, there is currently a limited evidence base surrounding the efficacy of LLIF surgery to correct sagittal deformity. Given the procedure utilises a lordotic cage to directly manipulate the anterior and middle columns, and patients are typically supplemented with posterior spinal fusion (PSF) with lordotic rods, it is hypothesised that LLIF has the potential to significantly correct lordosis and thereby PI-LL mismatch.
Our study aims to explore whether restoring spinopelvic harmony (PI-LL <10 degrees) could be an expected outcome of LLIF followed by PSF in patients who present with PI-LL mismatch, as well as whether surgery maintained that relationship in patients who presented in a balanced state. Additionally, we aim to determine whether the degree of sagittal plane correction could be predicted by patient factors including age, number of levels treated, range of extension on pre-operative imaging and supplemental treatment of L5/S1 via posterior lumbar interbody fusion (PLIF). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-605).
Methods
Patient population
We reviewed all cases of patients who underwent LLIF at the Royal Hobart Hospital and Calvary Healthcare Lenah Valley, between January 2012 and August 2019 by a single surgeon (A Dubey). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethics approval was obtained from the Tasmania Health and Medical Human Research Ethics Committee prior to undertaking the review (H0018149), with a waiver of consent approved by the ethics board.
The indications for surgery were adult patients with clinical manifestations of lumbar degenerative disc disease, including primarily scoliosis but also central canal stenosis, spondylosis and foraminal stenosis. Patients were considered for surgery if they demonstrated significant radiological evidence of degenerative disc disease from L1–5, with all demonstrating mechanical spinal column pain of significant severity. All patients failed a trial of non-operative management for greater than 6 months, including physiotherapy, analgesics, CT-guided corticosteroid injections and lifestyles adjustments. We excluded patients who had undergone previous lumbar instrumented fusion and those without adequate pre- and post-operative standing X-rays within 6 weeks of surgery.
Operative technique
The operative approach for LLIF has been detailed previously by our authors (38). In brief, the patient was positioned in the lateral decubitus position under general anaesthesia. A retroperitoneal transpsoas approach was utilised to access the lumbar spine under neuromonitoring and intraoperative imaging guidance. This was followed by complete discectomy with release of the contralateral annulus. An eight-degree lordotic cage, either 3D printed titanium (CASCADIATM, K2M, Leesburg, VA, USA), or polyetheretherketone (Aleutian, K2M) prior to 2017, was then inserted. Bone graft substitute (iFactor, Cerapedics, CO, USA) was used in all cases. Within one week, patients were positioned prone in lordosis on the Jackson table and supplemented with open PSF via pedicle screw fixation at all levels operated on (MESA, K2M). No further bony decompression or osteotomies were performed during the posterior approach, however, if clinical and radiological disc disease was present at the level of L5/S1, an L5/S1 PLIF (EIT, LifeHealthcare, 6° lordosis) was performed via bilateral laminotomies during the second stage surgery. The PSF was then extended to S1 or the pelvis via S2-alariliac (S2AI) screws.
Radiographic analysis
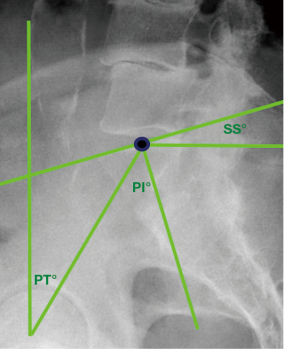
All pre- and post-operative X-rays were reviewed by two independent authors (M Asaid, A Cox) using Surgimap 2.2.15.15 spinal imaging software. Measurements were recorded for global LL, PI, and PI-LL mismatch (Figure 1). Lateral views were also examined for pelvic tilt (PT), sacral slope (SS), segmental lordosis, anterior and posterior disc heights, and LL in maximal extension where available. Range of extension was calculated as the difference in LL between neutral and extension films. AP images were reviewed for segmental Cobb angle at each treated level as well as the global Cobb angle of the lumbar spine (Figure 1).

Data analysis
Descriptive statistics were reported for the variables, noting mean, range, and standard deviation. Mean values between the assessors were recorded and used in the final analysis, with the intra-observer correlation coefficient calculated where applicable. Spinopelvic harmony was defined as PI-LL mismatch <10 degrees. Paired t-tests were used to compare pre- and post-operative outcomes that were normally distributed, and Wilcoxon signed-rank tests for those with skewed distributions. Logistic regression models were used to identify variable factors associated with patients achieving spinopelvic harmony, including the addition of an L5/S1 PLIF and number of levels treated. The outcome was the odds of achieving a PI-LL mismatch of <10 degrees. A P value of <0.05 was considered statistically significant.
Results
A total of 90 patients underwent LLIF across the two institutions during the study period. Following the relevant exclusions, 71 patients (50 female, 21 male) with a mean age of 66.7±8.6 years remained for radiographic analysis (Figure 2). A total of170 levels of LLIF were performed with 83.1% of patients undergoing multi-level procedures. The most commonly treated level was L3/4, with 95.8% of patients undergoing treatment at this level. Demographic and operative characteristics are detailed in Table 1.

Full table
With respect to the outcome of interest, a mean pre-operative PI-LL of 14.3 degrees and post-operative value of 13.2 degrees (P=0.43) was recorded. Of the 41 patients who presented in PI-LL mismatch, only 13 (31.7%) were restored to spinopelvic harmony post-LLIF procedure (Figures 2,3). 30 patients presented with PI-LL <10 degrees, and 25 of these (83.3%) maintained that relationship following LLIF surgery, including the illustrated case (Figure 1). Of the 5 patients who lost spinopelvic harmony, a mean post-operative PI-LL mismatch of 12.7 degrees was recorded, with no consistent surgical or radiological features observed across this subgroup. Intra-observer reliability was high within this study, with an intraclass correlation coefficient of 0.98 (P<0.001) for pre-operative PI-LL, and 0.97 (P<0.001) for post-operative PI-LL.
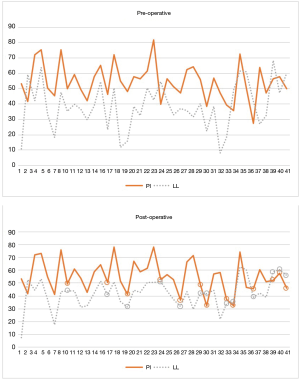
There was no significant difference in pre- and post-operative LL (Table 2), despite a statistically significant increase in segmental lordosis of 2.49° on average. When stratified by level (Table 3), segmental lordosis was maximally improved at L2/3 and the least at L4/5. At the L4-S1 levels, where typically the majority of LL is obtained, the mean lordosis reduced from 32.3° to 29.9° post-operatively (P=0.01) across the cohort. When L4/5±L5/S1 was treated, this difference was less pronounced (29.7° to 28.8°, P=0.62).

Full table
Full table
Anterior and posterior disc heights and coronal segmental and global Cobb angles all improved significantly post-LLIF surgery (Table 2). In regression analysis, age, sex, supplemental treatment of L5/S1 via PLIF, range of extension on pre-op imaging and number levels treated yielded no significant differences in ability to influence likelihood of establishing spinopelvic harmony (Table 4).

Full table
Discussion
In the constantly evolving landscape of spinal surgery, LLIF has established itself as a highly effective treatment option for lumbar spine pathology (25,26,30-32,36,37,39). In carefully selected patients, the procedure has consistently been demonstrated to result in improved clinical outcomes with a narrower side-effect profile than its anterior and posterior alternatives (39-43), likely attributable to its minimally invasive approach to the lumbar spine. Furthermore, a substantially larger interbody cage is placed during LLIF compared to posterior approaches, resulting in greater endplate contact and a sounder biomechanical environment for fusion, as well as the opportunity for deformity correction (20,25,30,44). Perhaps just as importantly as knowing the strengths of any procedure however, is understanding its limitations.
Until now, the role of LLIF alone in influencing PI-LL mismatch has not been well defined. The PI is a key fixed pelvic parameter, and defined as the angle between a line from the midpoint of the sacrum perpendicular to its endplate, and a line from this point to the axis of the femoral head (Figure 4) (3,5). It equates to the sum of the SS and PT. In degenerative scoliosis, a loss of lordosis is commonly observed which leads to compensatory mechanisms such as pelvic retroversion in an effort to maintain alignment, manifesting radiologically as an increase in PT (5,7,45). Whilst restoring LL is a common goal of spinal surgery, its relationship to the PI is the critical parameter which needs to be considered.
Based on asymptomatic individuals, and expanding on the earlier work of Boulay et al. (4), Schwab et al. derived the now widely accepted formula of LL = PI ±9° to define the LL required to achieve spinopelvic harmony (5). The same authors highlighted its clinical importance in a prospective multi-centre study (7), where the mismatch between PI and LL had the strongest radiographic correlation with disability and poor quality of life scores, a finding supported by further publications and biomechanical analyses (4,5,7-11). With the increased mechanical load on the lumbar spine as a result of PI-LL mismatch, Tempel et al. (12) demonstrated a direct correlation with clinically significant adjacent segment degeneration (ASD) post-spinal fusion, where of the 63 patients with PI-LL mismatch 77.8% required further surgery for ASD, compared to only 6.3% of those in spinopelvic harmony.
In our study of 71 patients with 170 levels treated, one of the largest subsets of LLIF patients to be reported on in the literature, we found no statistically significant differences in PI-LL from pre- to post-operatively. This finding may be a consequence of several factors. Firstly, the primary focus of our lateral approach was to provide indirect foraminal decompression and correct coronal deformity. Hence, although eight-degree lordotic cages were used, no further attempts were made during this stage to generate additional lordosis. Secondly, as well as further correction of coronal deformity, the goal of the PSF was to provide structural support to the lateral construct, improve load sharing and thus minimise cage subsidence. Osteotomies, significant screw compression and rods contoured to a pre-determined lordosis were therefore not utilised. Finally, although the inherent design of the Jackson table is one that enables lordosis, it is also possible that positioning during this stage could have been a factor.
With respect to LLIF, two recognised techniques have been demonstrated to have the potential to generate additional lordosis and thus increase the prospect of restoring spinopelvic harmony. Tempel et al. (25), in the only study to our knowledge to investigate LLIF and PI-LL mismatch, demonstrated a statistically significant restoration of PI-LL from 15.0° to 6.9° when Smith-Peterson-type or PSOs were frequently used prior to the lateral approach to facilitate distraction. Eleven of their 26 patients however experienced complications relating to surgery, confirming that such procedures are inherently associated with significantly increased risk (15,19,46). Expanding on the preliminary findings of Deukmedjian et al. (47,48), Manwaring et al. (49) successfully demonstrated that anterior column release (ACR) during the lateral approach can provide powerful lordotic correction equivalent to PSOs. This technique allowed for 30° lordotic cages to be inserted, improving global LL from a mean of 36.5° to 53.4° post-operatively. Again, notwithstanding the inevitable steep learning curve, by sectioning the anterior longitudinal ligament (ALL) there is an increased risk of devastating injury to the great vessels, segmental vessels, and bowel, all of which have been reported (50,51). Furthermore, an increased risk of ventral cage migration has been documented (49,52). Clearly, there are limitations to these powerful corrective mechanisms. Simply inserting a greater angled cage without ALL release is unlikely to have the same impact, with several centres reporting no significant global lordosis benefit with 10° cages (49,53), and a recent study by Otsuki et al. (54) demonstrating no significant radiological differences when comparing the use of 6° or 10° cages during lateral surgery.
It is well known that approximately 2/3rds of LL is obtained at L4–S1 (55), and as such this section is of greatest importance to the overall lumbar curvature. Interestingly, we found that when LLIF surgery was performed, most commonly at L3/4, the LL across this critical section reduced from 32.3° to 29.9°. It is therefore plausible that the distraction and modest segmental lordotic improvement achieved by treating higher levels (Table 3) can be offset by slight decreases of lordosis across the critical L4–S1 levels, resulting in an overall neutral radiological outcome. When L4/5 was treated via LLIF this difference was less pronounced, however the smallest segmental lordotic increase of 1.94° was also recorded at this level (Table 3). A recent review article by Winder et al. (56) highlighted similar rates of segmental L4/5 lordotic correction with LLIF across the literature, with difficulties with access and an intact ALL likely to limit the lordotic correction at this level. When an anterior approach was used for L4/5 treatment however and the ALL therefore incised, segmental lordosis improved by up to 8.3° (56,57).
Of the 30 patients in our cohort who presented in spinopelvic harmony, the majority (83.3%) maintained a PI-LL <10 degrees post-LLIF whilst also correcting coronal Cobb angles and disc heights. In addition to the standard selection criteria for LLIF surgery, we propose two key considerations when assessing suitable candidates pre-operatively. Based on our findings, it is clear the pre-operative evaluation of spinopelvic parameters is paramount to isolate patients with PI-LL <10 degrees. This subset of patients is most likely to retain spinopelvic harmony during the correction of coronal deformity in addition to the indirect neuroforaminal decompression that LLIF confers. Such examples are illustrated with clarity in Figure 1. However, where additional lordosis is required to correct PI-LL mismatch, strong consideration needs to be given to adjunctive procedures such as osteotomies during the posterior approach or ACR during the lateral approach, as LLIF followed by PSF in isolation is unlikely to be sufficient. As such, patients in this cohort need to be carefully counselled in terms of the extended operative approaches and the risks that this may carry. Ultimately, the authors recommend a case-by-case approach when discussing the role and extent that LLIF surgery plays in correcting spinal deformity based on the patient’s preoperative radiological characteristics.
The authors acknowledge the few limitations of this study. In particular, the potential for incomplete data and selection bias in a retrospective review is well recognised. Our cohort data is based on early postoperative imaging, thus, we acknowledge that cage subsidence and radiological changes may occur over time. This should be a focus in future studies to ascertain the long-term outcomes of LLIF surgery with respect to spinopelvic harmony, and although not a focus of our paper, the correlation with clinical outcomes would be of interest. Furthermore, this is a single surgeon series and may lack external validity, although congruence of our data set with previous reports is reassuring.
Conclusions
LLIF is a highly effective treatment for segmental coronal deformity whilst also improving disc heights and thereby providing indirect decompression. The prospect of restoring spinopelvic harmony with LLIF followed by PSF in imbalanced patients is low in the absence of adjunctive procedures. This study highlights the importance of preoperative evaluation of spinopelvic parameters, as patients that present with a PI-LL <10 degrees are likely to retain that relationship post-LLIF surgery and may be the best candidates when this approach is used in isolation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-605
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-605
Peer Review File: Available at http://dx.doi.org/10.21037/jss-20-605
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-605). CS 3rd serves as an unpaid editorial board member of the Journal of Spine Surgery from May 2019 to May 2021. CS 3rd reports that he is employed as a part-time consultant by LifeHealthcare Pty Ltd., and receives royalties for K2M Inc. for a product (Capri) unrelated to implants used in this study. No funding was sought from the company and they were not involved in the manuscript production. The authors have no other conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethics approval was obtained from the Tasmania Health and Medical Human Research Ethics Committee prior to undertaking the review (H0018149), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024-9. [Crossref] [PubMed]
- Lafage V, Schwab F, Patel A, et al. Pelvic Tilt and Truncal Inclination: Two Key Radiographic Parameters in the Setting of Adults with Spinal Deformity. Spine (Phila Pa 1976) 2009;34:E599-606. [Crossref] [PubMed]
- Legaye J, Duval-Beaupere G, Hecquet J, et al. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J 1998;7:99-103. [Crossref] [PubMed]
- Boulay C, Tardieu C, Hecquet J, et al. Sagittal alignment of spine and pelvis regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J 2006;15:415-22. [Crossref] [PubMed]
- Schwab F, Lafage V, Patel A, et al. Sagittal plane considerations and the pelvis in the adult patient. Spine (Phila Pa 1976) 2009;34:1828-33. [Crossref] [PubMed]
- Schwab F, Patel A, Ungar B, et al. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010;35:2224-31. [Crossref] [PubMed]
- Schwab FJ, Blondel B, Bess S, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine (Phila Pa 1976) 2013;38:E803-12. [Crossref] [PubMed]
- Senteler M, Weisse B, Snedeker JG, et al. Pelvic incidence–lumbar lordosis mismatch results in increased segmental joint loads in the unfused and fused lumbar spine. Eur Spine J 2014;23:1384-93. [Crossref] [PubMed]
- Glassman SD, Berven S, Bridwell K, et al. Correlation of Radiographic Parameters and Clinical Symptoms in Adult Scoliosis. Spine (Phila Pa 1976) 2005;30:682-8. [Crossref] [PubMed]
- Takemoto M, Boissiere L, Vital JM, et al. Are sagittal spinopelvic radiographic parameters significantly associated with quality of life of adult spinal deformity patients? Multivariate linear regression analyses for pre-operative and short-term post-operative health-related quality of life. Eur Spine J 2017;26:2176-86. [Crossref] [PubMed]
- Lafage R, Schwab F, Challier V, et al. Defining Spino-Pelvic Alignment Thresholds: Should Operative Goals in Adult Spinal Deformity Surgery Account for Age? Spine (Phila Pa 1976) 2016;41:62-8. [Crossref] [PubMed]
- Tempel ZJ, Gandhoke GS, Bolinger BD, et al. The Influence of Pelvic Incidence and Lumbar Lordosis Mismatch on Development of Symptomatic Adjacent Level Disease Following Single-Level Transforaminal Lumbar Interbody Fusion. Neurosurgery 2017;80:880-6. [Crossref] [PubMed]
- Di Martino A, Quattrocchi CC, Scarciolla L, et al. Estimating the risk for symptomatic adjacent segment degeneration after lumbar fusion: analysis from a cohort of patients undergoing revision surgery. Eur Spine J 2014;23 Suppl 6:693-8. [Crossref] [PubMed]
- Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J 2001;10:314-9. [Crossref] [PubMed]
- Popa I, Oprea M, Andrei D, et al. Utility of the pedicle subtraction osteotomy for the correction of sagittal spine imbalance. Int Orthop 2016;40:1219-25. [Crossref] [PubMed]
- Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine 2006;4:304-9. [Crossref] [PubMed]
- Bridwell KH, Lewis SJ, Lenke LG, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am 2003;85:454-63. [Crossref] [PubMed]
- Cho KJ, Bridwell KH, Lenke LG, et al. Comparison of Smith-Petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine (Phila Pa 1976) 2005;30:2030-7; discussion 2038. [Crossref] [PubMed]
- Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine (Phila Pa 1976) 2003;28:2093-2101. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal 2012;2012:456346. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989. [Crossref] [PubMed]
- Marchi L, Oliveira L, Amaral R, et al. Lateral interbody fusion for treatment of discogenic low back pain: minimally invasive surgical techniques. Adv Orthop 2012;2012:282068. [Crossref] [PubMed]
- Pimenta L, Turner AW, Dooley ZA, et al. Biomechanics of lateral interbody spacers: going wider for going stiffer. ScientificWorldJournal 2012;2012:381814. [Crossref] [PubMed]
- Tempel ZJ, Gandhoke GS, Bonfield CM, et al. Radiographic and clinical outcomes following combined lateral lumbar interbody fusion and posterior segmental stabilization in patients with adult degenerative scoliosis. Neurosurg Focus 2014;36:E11. [Crossref] [PubMed]
- Dahdaleh NS, Smith ZA, Snyder LA, et al. Lateral transpsoas lumbar interbody fusion: outcomes and deformity correction. Neurosurg Clin N Am 2014;25:353-60. [Crossref] [PubMed]
- Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 2013;38:1853-61. [Crossref] [PubMed]
- Anand N, Baron EM, Thaiyananthan G, et al. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech 2008;21:459-67. [Crossref] [PubMed]
- Caputo AM, Michael KW, Chapman TM, et al. Extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. J Clin Neurosci 2013;20:1558-63. [Crossref] [PubMed]
- Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331-7. [Crossref] [PubMed]
- Scherman DB, Rao PJ, Phan K, et al. Outcomes of direct lateral interbody fusion (DLIF) in an Australian cohort. J Spine Surg 2019;5:1-12. [Crossref] [PubMed]
- Caputo AM, Michael KW, Chapman TM Jr, et al. Clinical outcomes of extreme lateral interbody fusion in the treatment of adult degenerative scoliosis. ScientificWorldJournal 2012;2012:680643. [Crossref] [PubMed]
- Pereira EA, Farwana M, Lam KS. Extreme lateral interbody fusion relieves symptoms of spinal stenosis and low-grade spondylolisthesis by indirect decompression in complex patients. J Clin Neurosci 2017;35:56-61. [Crossref] [PubMed]
- Le TV, Vivas AC, Dakwar E, et al. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. ScientificWorldJournal 2012;2012:516706. [Crossref] [PubMed]
- Lee YS, Park SW, Kim YB. Direct lateral lumbar interbody fusion: clinical and radiological outcomes. J Korean Neurosurg Soc 2014;55:248-54. [Crossref] [PubMed]
- Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine 2012;16:329-33. [Crossref] [PubMed]
- Sharma AK, Kepler CK, Girardi FP, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech 2011;24:242-50. [Crossref] [PubMed]
- Sutterlin C, Luscombe J, Day J, et al. Lateral lumbar interbody fusion (LLIF): Technique and outcomes. Grande Medical Journal 2019. doi: https://doi.org/ [Crossref]
- Acosta FL, Liu J, Slimack N, et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine 2011;15:92-6. [Crossref] [PubMed]
- Rajaraman V, Vingan R, Roth P, et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg 1999;91:60-4. [PubMed]
- Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J 2009;9:623-9. [Crossref] [PubMed]
- Lee YS, Kim YB, Park SW, et al. Comparison of transforaminal lumbar interbody fusion with direct lumbar interbody fusion: clinical and radiological results. J Korean Neurosurg Soc 2014;56:469-74. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 2011;36:26-32. [Crossref] [PubMed]
- Saadeh YS, Joseph JR, Smith BW, et al. Comparison of Segmental Lordosis and Global Spinopelvic Alignment After Single-Level Lateral Lumbar Interbody Fusion or Transforaminal Lumbar Interbody Fusion. World Neurosurg 2019;126:e1374-8. [Crossref] [PubMed]
- Mehta VA, Amin A, Omeis I, et al. Implications of Spinopelvic Alignment for the Spine Surgeon. Neurosurgery 2012;70:707-21. [Crossref] [PubMed]
- Yang BP, Ondra SL, Chen LA, et al. Clinical and radiogrpahic outcomes of thoracic and lumbar pedicle subtraction osteotomy for fixed sagittal imbalance J Neurosurg Spine 2006;5:9-17. [Crossref] [PubMed]
- Deukmedjian AR, Le TV, Baaj AA, et al. Anterior longitudinal ligament release using the minimally invasive lateral retroperitoneal transpsoas approach: a cadaveric feasibility study and report of 4 clinical cases. J Neurosurg Spine 2012;17:530-9. [Crossref] [PubMed]
- Deukmedjian AR, Dakwar E, Ahmadian A, et al. Early outcomes of minimally invasive anterior longitudinal ligament release for correction of sagittal imbalance in patients with adult spinal deformity. ScientificWorldJournal 2012;2012:789698. [Crossref] [PubMed]
- Manwaring JC, Bach K, Ahmadian AA, et al. Management of sagittal balance in adult spinal deformity with minimally invasive anterolateral lumbar interbody fusion: a preliminary radiographic study. J Neurosurg Spine 2014;20:515-22. [Crossref] [PubMed]
- Murray G, Beckman J, Bach K, et al. Complications and neurological deficits following minimally invasive anterior column release for adult spinal deformity: a retrospective study. Eur Spine J 2015;24 Suppl 3:397-404. [Crossref] [PubMed]
- Uribe JS, Deukmedjian AR. Visceral, vascular, and wound complications following over 13,000 lateral interbody fusions: a survey study and literature review. Eur Spine J 2015;24 Suppl 3:386-96. [Crossref] [PubMed]
- Xu DS, Paluzzi J, Kanter AS, et al. Anterior Column Release/Realignment. Neurosurg Clin N Am 2018;29:427-37. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Maintenance of Segmental Lordosis and Disk Height in Stand-alone and Instrumented Extreme Lateral Interbody Fusion (XLIF). Clin Spine Surg 2017;30:E90-8. [Crossref] [PubMed]
- Otsuki B, Fujibayashi S, Takemoto M, et al. Analysis of the Factors Affecting Lumbar Segmental Lordosis After Lateral Lumbar Interbody Fusion. Spine (Phila Pa 1976) 2020;45:E839-46. [Crossref] [PubMed]
- Barrey C, Darnis A. Current strategies for the restoration of adequate lordosis during lumbar fusion. World J Orthop 2015;6:117-26. [Crossref] [PubMed]
- Winder MJ, Gambhir S. Comparison of ALIF vs. XLIF for L4/5 interbody fusion: pros, cons, and literature review. J Spine Surg 2016;2:2-8. [Crossref] [PubMed]
- Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379-86. [Crossref] [PubMed]



