Greater than 5-year follow-up of outpatient L4−L5 lumbar interspinous fixation for degenerative spinal stenosis using the INSPAN device
Introduction
Lumbar spine stenosis is a highly prevalent condition that often results from degenerative disc disease, degenerative spondylolisthesis, arthritis, and facet arthrosis which are major contributory pathologies for lower back pain (1). In patients greater than 60 years of age, lumbar stenosis can lead to impaired ambulation with increased morbidity secondary to lower back pain and lower extremity neuropathy (2).
Interspinous process device (IPD) could improve the central canal area in up to 18% in cadaveric spine studies (3). IPDs were approved for patient use at the beginning of the century (3) and introduced as a less invasive surgical alternative. Several complications associated with the use of interspinous spacers have included device dislocation or malposition, spinous process fractures, infection, hematoma, erosion of the spinous process, and neurological sequelae (4,5). Revision, reoperation rates for IPD range from 4.6% to as high as 85% in studies (5,6). There is a paucity of data on a single device that has been used for both fusions and stenosis. Authors aim to demonstrate the long-term outcomes of interspinous fixation at L4-5 for degenerative spinal stenosis using the InSpan device. We present the study in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jss-20-547).
Methods
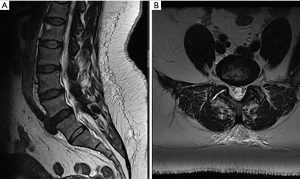
The database of a single spine surgeon was retrospectively reviewed over the last 5 years. IRB approval was granted WIRB 20181251 for this study and informed consent was obtained. The charts of 122 patient undergoing Interspinous Distraction, Fixation and Fusion using InSpan (InSpan LLC) in the outpatient setting were reviewed. The mechanism of action of the InSpan device (Figure 1) is interlaminar, interspinous decompression, this device has no FDA recalls reported. Features of this device include a rigid interlocking hub, low profile shape which conforms to anatomy, staggered spike to prevent fracture and optimize fixation. Patients were only considered for surgery after they failed conservative management for at least three months. Indications for surgery included spinal stenosis, degenerative disc disease (Figure 2). Exclusion criteria for this study included acute severe trauma, fractures, malignancy, infection, unstable chronic medical illnesses, prior lumbar fusions and a BMI >42 (7). All patients were assessed preoperatively and narcotics were discontinued (8). Patients with chronic but stable medical conditions, including hypertension, diabetes mellitus, asthma, hypercholesterolemia, and heart disease were medically cleared by their family practitioner and/or cardiologist where applicable.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board (No. WIRB 20181251), and because of the retrospective nature of the research, the requirement for informed consent was waived.
Summary of operative technique
Steps in the Less Exposure Surgery (LES) Interspinous Fixation included preparation, positioning, incision, fascial opening, dissection, retractors, bone identification, deperiostization, decompression, microdiscectomy, and closure.
The patient was brought to the operating room and placed in the prone position on the Wilson frame. All bony prominences were well padded. After all appropriate anesthesia monitors were attached, the patient underwent general endotracheal anesthesia and the lumbar spine was prepped and draped in a standard sterile surgical fashion and the preoperative surgical site was marked was visible ink in the operative field. The Wilson frame was elevated to open the spinous processes of the affected level.
Step 1: Access/Exposure
Using standard surgical landmarks, the pedicles were identified and the position was confirmed using a 22G needle (9) and AP and lateral intraoperative fluoroscopy. An approximately 1.0-inch midline incision was made at the target level over the spinous process for single level decompression. Dissection using electrocautery on the left side of the spinous process was performed, allowing a cuff of tissue for anatomic closure. The rectus spinal muscles were elevated laterally and dissected in the subperiosteal and avascular plane of the lamina of the superior and inferior vertebral levels on the left.
Step 2: Decompression
The left superior vertebrae inferior facets and lamina were identified, and a #15 blade was used to make an incision to the lateral aspect of the ligamentum flavum. A curette was used to take down ligamentum flavum off the underside of the superior vertebrae lamina and the posterior side of the inferior vertebrae lamina. The curette was used to separate the ligamentum flavum from the facet capsule. Kerrison Ronguers were used to perform a left hemilaminotomy, foraminotomy, and partial facetectomy until the surgeon was satisfied that the lateral recess was adequately decompressed. Hemostasis was achieved
Step 3: Discectomy
Careful retraction of the nerve root was performed medially using a nerve root retractor with the ligamentum flap completed to show the affected disc. A microdiscectomy was performed to remove the soft, loose and unhealthy disc using multiple pituitaries. The disc space was irrigated to remove any remaining free disc fragments.
Step 4: Repeated on the right for bilateral
Steps 1−3 were repeated on the right if the procedure was for bilateral decompression with or without microdiscectomy. If the procedure was only unilateral, then just the affected side was treated.
Step 5:
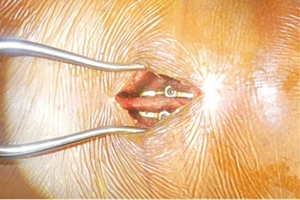
To unload the facets, distraction across the affected level performed and measuring performed for an appropriate size interspinous fixation device. The appropriately sized InSpan device (InSpan Inc, Malden, MA, USA) was then placed at L4-5disc space (Figure 3). To ensure stability the device is locked with set screws once squeezed together.
Step 6: Closure
Hemostasis was achieved using bone wax and surgi-flo to control bleeding. The wounds were then irrigated copiously. Final fluoroscopy was taken AP and lateral fluoroscopic views to confirm position. A #1 Vicryl figure-of-eight was then placed to re-approximate the muscle and fascia. A 2-0 Vicryl was placed in the subcutaneous tissue and then a 3-0 monocryl was placed in the skin. A dry sterile dressing was applied.
Discharge and follow-up
Patients were discharged using the standard outpatient protocol of completing surgery after being deemed oriented and neurologically intact by the anesthesiologist and operating surgeon (10-13). Outpatient postoperative instructions were discussed with patients and caregivers with written copies provided (7,10-13).
Statistical analysis
Values are expressed as counts or means ± standard error as appropriate. Intergroup comparisons were made using a t-test. Data were analyzed using the SPSS statistical software version 22 (IBM Corp., New York, USA). Tests were considered significant if P<0.05.
Results
122 surgical cases of lumbar decompression with interspinous fixation, spanning between the timeframe of September 2011 to October 2016. A total of 56 patients had instrumentation at L4−L5. Total female population was 46%. The median age of the patients included in the population was 50.9±10.7 years with a median BMI of 24.8±11.4 kg/m2.
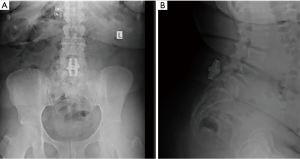
Mean VAS back scores decreased from 8.1±1.2 to 1.5±1.1 at two years follow-up, P=0.001. Preoperative ODI scores improved from 42.9±14.3 to 14.8 ±5.1 at two-year follow-up, P=0.001. The mean EBL and surgeon time was 40±15 mL and 45±15 minutes, respectively. Figure 4 shows postoperative X-ray with adequate intralaminar distraction.
Over the 5-year period, there was a total of 1 revision case with removal of InSpan and open hemilaminectomy decompression. We had no spinal fractures, implant failures, implant dislocation or deaths over the five-year period
Discussion
The interest in MIS continues to increase for patients, surgeons and commercial industries alike. MIS provides surgeons with the ability to intervene in the patient’s disease process and provide adequate symptomatic relief with shorter recovery times.
In a systematic review study by Zhu et al. (14) 26 papers were reviewed, with eight more eligible articles found by means of reviewing the references of those studies. There were 13 kinds of interspinous fixation devices available in such clinical or biomechanical research. Clinical evidence revealed improvement in ODI scores up to 92.5% (15) in one study and fusion ranging from 93.4% to 100% (16-19). One study noted spinous fractures in 33.3% (20).
Our study was a single center, single surgeon five-year follow-up of a large cohort receiving interspinous fixation after lumbar decompression. Patients demonstrated improved outcomes with a single revision. The authors of this study recognize that there were several limitations to the study design. Foremost, the results included in this analysis are comprised of the experiences of a single surgeon. It would be ideal to evaluate outcomes from several different operators with varying degrees of experience using this technique. Further evidence is required to determine the efficacy of the surgical technique in patients who have a higher BMI. Future studies should take such limitations into consideration when studying lumbar decompression and interspinous fixation and consider incorporating other objective measurements to determine the overall efficacy of LES such as cost per case and days to return to work.
Conclusions
This study demonstrates long term results with improved outcomes in patients who underwent interspinous distraction decompression in the outpatient setting using the INSPAN IPD at L4−L5 for Degenerative Spinal Stenosis. There was one revision converted to hemilaminectomy. There were no complications, blood transfusions or implant failures.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jss-20-547
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jss-20-547
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form(available at: http://dx.doi.org/10.21037/jss-20-547). KRC is a shareholder in and receives other benefits from KICVentures. None of the other authors (FJRP, AB and JAS) or any member of his or her immediate family has funding or commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board (NO. WIRB 20181251), and because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boden SD, Riew KD, Yamaguchi K, et al. Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg Am 1996;78:403-11. [Crossref] [PubMed]
- Suri P, Rainville J, Kalichman L, et al. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA 2010;304:2628-36. [Crossref] [PubMed]
- Richards JC, Majumdar S, Lindsey DP, et al. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine (Phila Pa 1976) 2005;30:744-9. [Crossref] [PubMed]
- Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine 2006;4:463-71. [Crossref] [PubMed]
- Gazzeri R, Galarza M, Neroni M, et al. Failure rates and complications of interspinous process decompression devices: a European multicenter study. Neurosurg Focus 2015;39:E14. [Crossref] [PubMed]
- Epstein NE. A review of interspinous fusion devices: high complication, reoperation rates, and costs with poor outcomes. Surg Neurol Int 2012;3:7. [Crossref] [PubMed]
- Chin KR, Coombs AV, Seale JA. Feasibility and patient-reported outcomes after outpatient single-level instrumented posterior lumbar interbody fusion in a surgery center: preliminary results in 16 patients. Spine (Phila Pa 1976) 2015;40:E36-42. [Crossref] [PubMed]
- Lawrence JT, London N, Bohlman HH, et al. Preoperative narcotic use as a predictor of clinical outcome: results following anterior cervical arthrodesis. Spine (Phila Pa 1976) 2008;33:2074-8. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Kubik J, et al. Avoidance of Wrong Level Surgery in the Lumbar Spine: A Technical Report. J Spine 2015;4:257.
- Chin KR, Pencle FJ, Coombs AV, et al. Lateral Lumbar Interbody Fusion in Ambulatory Surgery Centers: Patient Selection and Outcome Measures Compared With an Inhospital Cohort. Spine (Phila Pa 1976) 2016;41:686-92. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Coombs AV, et al. Clinical Outcomes With Midline Cortical Bone Trajectory Pedicle Screws Versus Traditional Pedicle Screws in Moving Lumbar Fusions From Hospitals to Outpatient Surgery Centers. Clin Spine Surg 2017;30:E791-7. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Coombs AV, et al. Eligibility of Outpatient Spine Surgery Candidates in a Single Private Practice. Clin Spine Surg 2017;30:E1352-8. [Crossref] [PubMed]
- Chin KR, Pencle FJR, Seale JA, et al. Clinical Outcomes of Outpatient Cervical Total Disc Replacement Compared With Outpatient Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2017;42:E567-74. [Crossref] [PubMed]
- Zhu L, Yin J. Interspinous fusion device: A systematic review of clinical and biomechanical evidence. Advances in Mechanical Engineering 2016;8:1687814016680517. [Crossref]
- Kim HJ, Bak KH, Chun HJ, et al. Posterior interspinous fusion device for one-level fusion in degenerative lumbar spine disease: comparison with pedicle screw fixation - preliminary report of at least one year follow up. J Korean Neurosurg Soc 2012;52:359-64. [Crossref] [PubMed]
- Neo M, Fujibayashi S, Yoshida M, et al. Spinous process plate fixation as a salvage operation for failed anterior cervical fusion. Technical note. J Neurosurg Spine 2006;4:78-81. [Crossref] [PubMed]
- Iwatsuki K, Yoshimine T, Yoshimura K, et al. Intractable Chronic Low-Back Pain Caused by Ligamentopathia Treated Using a Spinous Process Plate (S-plate). Clin Med Insights Arthritis Musculoskelet Disord 2010;3:1-5. [Crossref] [PubMed]
- Tomii M, Itoh Y, Numazawa S, et al. Spinous process plate (S-plate) fixation after posterior interbody fusion for lumbar canal stenosis due to spondylolisthesis. Neurosurg Rev 2013;36:139-43. [Crossref] [PubMed]
- Whitehill R, Schmidt R. The posterior interspinous fusion in the treatment of quadriplegia. Spine (Phila Pa 1976) 1983;8:733-40. [Crossref] [PubMed]
- Kim DH, Shanti N, Tantorski ME, et al. Association between degenerative spondylolisthesis and spinous process fracture after interspinous process spacer surgery. Spine J 2012;12:466-72. [Crossref] [PubMed]