Clinical presentation and diagnosis of acute postoperative spinal implant infection (PSII)
Introduction
Early or acute postoperative infection following spine surgery is a potentially serious complication that often requires prolonged treatment and is associated with a poor outcome for the patient (1). Moreover, in common with other septic complications in the field of orthopedic and trauma surgery, it represents a considerable socioeconomic burden (2,3). The suspicion of early infection may be difficult to confirm, as not all cases involve pain or elevated laboratory parameters of inflammation. In addition, diagnostic imaging is hampered by implants and the artifacts they cause. In such cases it is difficult to differentiate hematoma, edema, and infection on a postoperative magnetic resonance imaging (MRI) scan (4). Furthermore, diagnostic aspiration and microbiological analysis alone, as practiced in infections following joint replacement, is not considered standard (5). The expert opinion emerging from the Second International Consensus Meeting (ICM) 2018 was that should surgical intervention become necessary in early or acute infection, wound revision with debridement and retention of the implant is recommended. On the one hand, leaving the implant in place includes the risk of chronic infection with implant loosening or even infectious non-union, but on the other hand removal of the implant usually leads to an unstable situation that may also culminate in pseudarthrosis, pain and neurologic disorders (6). Thus, there are various options for the treatment of early/acute postoperative infection, but no guidelines or specific management advice.
Epidemiology
The first description of superficial and deep wound infection was published by Turnbull in 1953 (7). The incidence of postoperative infections following surgical interventions on the spine was reported in the literature as 0.7–8.5% (8). Superficial infections are distinguished from deep subfascial infections (7). Depending on comorbidity, the extent, surgical access, and duration of the surgery, and whether the operation is a first or subsequent procedure, the risk of infection is as high as 8.5–12% for implant-based interventions (6,9,10).
Risk factors
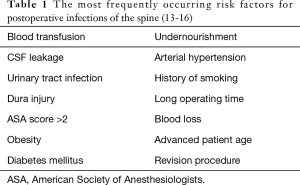
Schuster et al. postulated that implant-based interventions involve no extra risk (11), but a number of different studies have shown that this is not the case (12). Various factors have been demonstrated to elevate the risk of peri-spinal implant infection (PSII). These include, among others, blood transfusion, leakage of cerebrospinal fluid, urinary tract infection, injury of the dura mater, an American Society of Anesthesiologists (ASA) score >2, obesity, diabetes mellitus, and surgical revision (13). An overview can be found in Table 1.
Full table
Diagnostics
The clinical symptoms are the primary indicator for the presence of infection. These include fever, pain, and neural symptoms (e.g., because of local abscesses). Elevated inflammatory laboratory parameters are a clear sign of infection. The time at which the symptoms first occur may help to pinpoint the site of infection. Superficial infections often arise within 30 days after surgery, while deeper infections may occur early or at any later time (12). There is an established classification of infections after joint replacement of the large joints—early (≤3 months), delayed (>3–24 months), and late (>24 months) (17,18)—but no such classification of infections according to the time of occurrence has yet been widely accepted for spinal surgery.
Clinical manifestations
Early diagnosis and treatment of postoperative surgical site infection (SSI) is impossible if the risk of this complication is not borne in mind. Although SSI is rare, its incidence varies greatly depending on the type of intervention and on the patient clientele. No patient collective has ever been described with no infections at all following surgical interventions on the spine. Early diagnosis of wound infection is crucial for the patient’s recovery, and this aspect of postoperative follow-up must be firmly anchored in clinical routine. Besides anamnesis, clinical examination is the first step in any case of suspected wound infection. It is common for the findings (pain at rest, on motion, pressure pain; abnormal warmth; local erythema; circumscribed swelling; increased secretion; impaired articular function; and fever) to range anywhere between inconspicuous or concealed symptoms and the complete spectrum of inflammatory signs or septic shock. The diagnosis of infection subsequent to spinal surgery is hampered by the fact that the clinical signs can equally be positive during a “normal” postoperative course (19). Patients without infection may well display pain and swelling, or occasionally even abnormal warmth, erythema, and functional impairment (Table 2).

Full table
Investigation begins with the patient’s account of their symptoms. For an objective valuation of the anamnesis it is helpful to observe the patient during this consultation carefully. Second, the possibly infected area of the body should be inspected and palpated. The patient must be asked about the nature of the pain, where it occurs, and when (at rest, on motion, persistently, at night). Pain that initially decreases after the surgery but then increases is not generally a normal postoperative finding.
Postoperative wound inspection
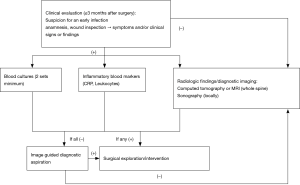
In patients under suspicion for an early SSI, the bandage is removed and the soft tissues are examined on each day after surgery. The bandage itself should be inspected and its state documented. A dry bandage does not exclude surgical infection. Local erythema, swelling, and/or secretion must be recorded. Objectively quantifiable changes, e.g., erythema increases, should be documented in centimeters. The changing extent of the discoloration can be monitored by marking the margin of the affected area. With respect to sterility, it is crucial to observe the postoperative course closely, with examination several times daily if required. Whenever clinical signs of inflammation are found in the postoperative phase, the presence of an infection must be assumed; only in this way postoperative SSI can be detected. Postoperative urinary tract infection and pneumonia must be excluded by means of the appropriate diagnostic procedures. If two of the four classical parameters of infection (erythema, pain, abnormal warmth, swelling) are present as new findings requiring specific treatment (19), maybe accompanied by renewed secretion from the wound or demonstration of sepsis by postoperative blood tests (Figure 1) the working diagnosis must be manifest early SSI.
Laboratory parameters
The presence of pus is a certain sign of infection and may be manifested by an abscess, empyema, or a fistula. Another definite sign of infection is septicemia. Pronounced clinical symptoms do not always go hand in hand with large-scale tissue destruction, and equally it cannot be assumed that less conspicuous clinical findings mean only minor damage. Because the clinical signs of postoperative SSI are the only signs that can definitely be relied upon, laboratory findings play a subsidiary role. In general, elevated C-reactive protein (CRP) and leukocyte concentrations can be found a few days after surgery and are “normal” in the course of reparative processes. Fujita et al. investigated the postoperative CRP of 948 patients who had undergone spondylodesis and found a second rise in CRP level (CRP-SR) in 107 cases. Thirty-eight (35%) of the patients with CRP-SR had developed a SSI. The remainder either had other infections (urinary tract infection, pneumonia) or the reason for the CRP-SR was not identified. Among the patients with CRP-SR, the best diagnostic cut-off value for detection of SSI was 30.4 mg/L (21). In this scenario the preoperative CRP level can, if required, be used as a reference value. Blood cultures are indispensable to establish the diagnosis and with it the necessary treatment procedures, as early identification of the pathogen with a resistogram enables the appropriate antibiotic therapy.
Imaging
The imaging modalities generally used to confirm a suspected infection are radiography, CT, and MRI. Standard radiographs usually show no abnormalities in the first 3 weeks despite the infection. If at all, gas-producing bacteria may form discernable pockets of gas in the soft tissues, which might be visible in X-ray images (20,22). Radiographs obtained later in the course of infection may visualize bony endplate destruction, bone resorption, or osteolysis in the interface between bone and implants. MRI is currently the gold standard for detection of postoperative infection after spinal interventions, even in the early phase. It can depict not only epidural abscesses but also other destructive and infectious processes in the intervertebral disks and soft tissues (23). An infection may affect the soft tissues of the “surgical bed”, without involving the spinal structures. In such a case MRI with and without contrast medium is strongly recommended (24). Contrast enhancement increases the specificity for acute infection detection, facilitates diagnosis in the absence of severe edema, and is particularly helpful in delineation of epidural extension and identification of abscesses (25). MRI of a spinal epidural abscess yields a signal of low or moderate intensity in T1-weighted sequences and high or moderate intensity in T2-weighted sequences. The fluid component of an abscess is normally extremely hyperintense on T2-weighted images and hypointense on T1-weighted images (26). Although MRI is the most effective modality for determining the fluid component of abscesses and osseous edema, CT is the superior method for depiction of the bony destruction (27,28). However, metal artifacts always hamper assessment of the area around the implants (29,30). Furthermore, hematoma cannot always be confidently distinguished from pus etc. in the early postoperative phase (4).
Microbiology
To our knowledge, no study has yet distinguished the pathogen spectrum of acute infections following surgical interventions on the spine from that of chronic infections. For this reason, we refer here to the spectrum of pathogens in postoperative spinal infections in general. The pathogens most frequently demonstrated in implant-associated infections, both on sonication and on examination of tissue samples, are coagulase-negative staphylococci (CNS). Besides this, in general Staphylococcus epidermidis is found most often, followed by Propionibacterium acnes and Staphylococcus aureus (31-34). Interestingly, patients with recurrent Staphylococcus aureus bacteriuria and bacteremia have a higher rate of spinal infections than patients with only Staphylococcus aureus bacteremia caused by retrograde dissemination through the pelvic venous and lymphatic vessels that are connected to the intraspinal plexus (35).
Antiseptics are effective against these pathogens and are thus recommended for intraoperative use (36). Recently published studies have shown that sodium hypochlorite is superior to chlorhexidine with regard to destruction of the biofilm (37). Since some bacteria form an immature biofilm in the 4-week phase directly after primarily contaminating surgery already (38), sodium hypochlorite must be recommended for biofilm-forming bacterial species even in revision surgeries of early postoperative SSIs. The fact that sodium hypochlorite is a fast-acting antiseptic is an additional advantage.
Treatment recommendation and perspective
On the basis of the criteria outlined above, the following algorithm (Figure 2) can be proposed: Newly occurring pain that is accompanied by conspicuous findings at the incision site, e.g., secretion or poor wound healing, should, if backed up by the radiological signs, be interpreted as infection. Surgery is then indicated to shorten the otherwise prolonged treatment process. The operative intervention begins with exploration of the site and sampling of tissue for microbiological examination. Three to five samples should be obtained (5), followed by painstaking debridement concluding with antiseptic lavage or, if required, jet lavage (39). On the basis of recent findings, local application of vancomycin is recommended (1,16,40). Finally, a drain is inserted and the wound is closed with cutaneous sutures. Non-loosened implants should be left in place when an early infection is treated, but loosened implants should be replaced. If an early infection persists after several revisions, one single replacement of the implant can be considered. Persisting infection often necessitates a “second-look” operation. If there is a large soft-tissue defect, elimination of the infection may have to be followed by plastic surgery. In the event of severe, practically uncontrollable infection, long-term suppression may be required until a status is achieved that permits removal of the implant(s). The same applies to a patient who is not conditional for surgery in general. In this case, sonographically or CT-guided aspiration or even drain insertion may help to harvest samples for identification of the pathogen or to relieve an abscess. In implant-preserving procedures, the Pro Implant Foundation (Trampuz et al.) recommends administration of antibiotics with biofilm activity for 2 weeks i.v. and then 10 weeks p.o. (16). In the absence of implants, or if the implant(s) can be removed in entirety, the current recommendation is that the oral antibiotics should be given for 4 weeks.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection (PSII)” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-587). The series “Postoperative Spinal Implant Infection (PSII)” was commissioned by the editorial office without any funding or sponsorship. TZ reports personal fees from Medtronic, outside the submitted work. PS reports personal fees from Medtronic, personal fees from SpineArt, grants from German Ministry of Economy, grants from German Spine Society, personal fees from Medacta, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou J, Wang R, Huo X, et al. Incidence of Surgical Site Infection After Spine Surgery: A Systematic Review and Meta-analysis. Spine (Phila Pa 1976) 2020;45:208-16. [Crossref] [PubMed]
- Müller M, Trampuz A, Winkler T, et al. The Economic Challenge of Centralised Treatment of Patients with Periprosthetic Infections. Z Orthop Unfall 2018. Epub ahead of print. [Crossref] [PubMed]
- Calderone RR, Garland DE, Capen DA, et al. Cost of medical care for postoperative spinal infections. Orthop Clin North Am 1996;27:171-82. [PubMed]
- Chahoud J, Kanafani Z, Kanj SS. Surgical site infections following spine surgery: eliminating the controversies in the diagnosis. Front Med (Lausanne) 2014;1:7. [Crossref] [PubMed]
- Renz N, Müller M, Perka C, et al. Implant-associated infections - Diagnostics. Chirurg 2016;87:813-21. [Crossref] [PubMed]
- Divi SN, Kepler CK, Boody BS, et al. Consensus on Implants in Infections After Spine Surgery. Clin Spine Surg 2020;33:163-71. [Crossref] [PubMed]
- Turnbull F.. Postoperative inflammatory disease of lumbar discs. J Neurosurg 1953;10:469-73. [Crossref] [PubMed]
- Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine (Phila Pa 1976) 1997;22:2444-50; discussion 2450-1. [Crossref] [PubMed]
- Anderson PA, Savage JW, Vaccaro AR, et al. Prevention of Surgical Site Infection in Spine Surgery. Neurosurgery 2017;80:S114-23. [Crossref] [PubMed]
- Buric J, Berjano P, Damilano M.. Severe Spinal Surgery Infection and Local Ozone Therapy as Complementary Treatment: A Case Report. Int J Spine Surg 2019;13:371-6. [Crossref] [PubMed]
- Schuster JM, Rechtine G, Norvell DC, et al. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976) 2010;35:S125-37. [Crossref] [PubMed]
- Lazennec JY, Fourniols E, Lenoir T, et al. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res 2011;97:S107-16. [Crossref] [PubMed]
- Meng F, Cao J, Meng X. Risk factors for surgical site infections following spinal surgery. J Clin Neurosci 2015;22:1862-6. [Crossref] [PubMed]
- Lehner B, Akbar M, Beckmann NA. Infections after reconstructive spinal interventions: How do I deal with them? Orthopade 2018;47:288-95. [Crossref] [PubMed]
- Adhikari P, Nabiyev VN, Bahadir S, et al. Does the Application of Topical Intrawound Vancomycin Powder Affect Deep Surgical Site Infection and the Responsible Organisms after Spinal Surgery?: A Retrospective Case Series with a Historical Control Group. Asian Spine J 2020;14:72-8. [Crossref] [PubMed]
- Prinz V, Bayerl S, Renz N, et al. High frequency of low-virulent microorganisms detected by sonication of pedicle screws: a potential cause for implant failure. J Neurosurg Spine 2019;31:424-9. [Crossref] [PubMed]
- Dapunt U, Bürkle C, Günther F, et al. Surgical site infections following instrumented stabilization of the spine. Ther Clin Risk Manag 2017;13:1239-45. [Crossref] [PubMed]
- Li C, Renz N, Trampuz A.. Management of Periprosthetic Joint Infection. Hip Pelvis 2018;30:138-46. [Crossref] [PubMed]
- Bühler M, Engelhardt M, Schmidt HGK. Septische postoperative Komplikationen, Atlas für Unfallchirurgen und Orthopäden. Springer, Wien, 2003.
- Chaudhary SB, Vives MJ, Basra SK, et al. Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med 2007;30:441-51. [Crossref] [PubMed]
- Fujita R, Takahata M, Kokabu T, et al. Retrospective study to evaluate the clinical significance of a second rise in C-reactive protein level following instrumented spinal fusion surgery. J Orthop Sci 2019;24:963-8. [Crossref] [PubMed]
- Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature. Rev Infect Dis 1979;1:754-76. [Crossref] [PubMed]
- McDermott H, Bolger C, Humphreys H. Postprocedural discitis of the vertebral spine: challenges in diagnosis, treatment and prevention. J Hosp Infect 2012;82:152-7. [Crossref] [PubMed]
- Post MJ, Sze G, Quencer RM, et al. Gadolinium-enhanced MR in spinal infection. J Comput Assist Tomogr 1990;14:721-9. [Crossref] [PubMed]
- Dagirmanjian A, Schils J, McHenry M, et al. MR imaging of vertebral osteomyelitis revisited. AJR Am J Roentgenol 1996;167:1539-43. [Crossref] [PubMed]
- Lang IM, Hughes DG, Jenkins JP, et al. MR imaging appearances of cervical epidural abscess. Clin Radiol 1995;50:466-71. [Crossref] [PubMed]
- Kanayama M, Hashimoto T, Shigenobu K, et al. MRI-based Decision Making of Implant Removal in Deep Wound Infection After Instrumented Lumbar Fusion. Clin Spine Surg 2017;30:E99-E103. [Crossref] [PubMed]
- Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985;157:157-66. [Crossref] [PubMed]
- Shellock FG. MR imaging and cervical fixation devices: evaluation of ferromagnetism, heating, and artifacts at 1.5 Tesla. Magn Reson Imaging 1996;14:1093-8. [Crossref] [PubMed]
- Shellock FG. Metallic neurosurgical implants: evaluation of magnetic field interactions, heating, and artifacts at 1.5-Tesla. J Magn Reson Imaging 2001;14:295-9. [Crossref] [PubMed]
- Bürger J, Akgün D, Strube P, et al. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J 2019;28:768-74. [Crossref] [PubMed]
- Shifflett GD, Bjerke-Kroll BT, Nwachukwu BU, et al. Microbiologic profile of infections in presumed aseptic revision spine surgery. Eur Spine J 2016;25:3902-7. [Crossref] [PubMed]
- Parchi PD, Evangelisti G, Andreani L, et al. Postoperative Spine Infections. Orthop Rev (Pavia) 2015;7:5900. [Crossref] [PubMed]
- Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 2010;35:1218-24. [Crossref] [PubMed]
- Kim MJ, Koo HM, Lee WJ, et al. Development of Epidural and Paraspinal Abscesses after Insufficient Evaluation and Treatment of Acute Pyelonephritis Caused by Staphylococcus aureus. Korean J Fam Med 2016;37:299-302. [Crossref] [PubMed]
- Röhner E, Hoff P, Pfitzner T, et al. Limited use of antiseptics in septic surgery. J Invest Surg 2012;25:311-6. [Crossref] [PubMed]
- Röhner E, Jacob B, Böhle S, et al. Sodium hypochlorite is more effective than chlorhexidine for eradication of bacterial biofilm of staphylococci and Pseudomonas aeruginosa. Knee Surg Sports Traumatol Arthrosc 2020;28:3912-8. [Crossref] [PubMed]
- Janz V, Löchel J, Trampuz A, et al. Risk factors and management strategies for early and late infections following reconstruction with special tumour endoprostheses. Orthopade 2020;49:142-8. [Crossref] [PubMed]
- Hawellek T, Beil FT, Hubert J. Revision surgery in acute periprosthetic knee joint infections. Oper Orthop Traumatol 2018;30:309-20. [Crossref] [PubMed]
- Li S, Rong H, Zhang X, et al. Meta-analysis of topical vancomycin powder for microbial profile in spinal surgical site infections. Eur Spine J 2019;28:2972-80. [Crossref] [PubMed]