
Stereotactic navigation in anterior cervical spine surgery: surgical setup and technique
Introduction
Anterior cervical spine surgery requires detailed knowledge of surgical anatomy in order to avoid iatrogenic injury to critical structures such as the esophagus, and neurovascular elements such as the vertebral artery, spinal cord, and nerve roots which lie in close proximity to where the surgeon and his or her instrumentation is working. Although not a substitute for an understanding of the anatomy, stereotactic intraoperative imaging is an evolving tool that can assist the surgeon when operating on the cervical spine. It is particularly helpful in cases where normal anatomic landmarks are absent or altered such as deformity or trauma, revision surgery, morbid obesity, ossification of the posterior longitudinal ligament or in cases where minimally invasive approaches are desired (1,2).
The concept of stereotactic operations is not a new one, as this method has been used for intracranial surgery purposes since the beginning of the century. The adoption of computer assisted navigation in spine surgery began in the 1990’s and has been an evolving tool. In its early stages, this technology relied on “frame-based stereotaxis”, similar to that used in intracranial procedures (3). A pre-operative CT scan was obtained and this was used to create a three-dimensional (3D) representation of the vertebral levels of interest within the navigation unit. Intraoperatively, a dynamic frame with a rigid body had to be placed at each spinal level of intended work. A 3D pointer and optical localizer were then used to mark various anatomic points at each level so that the patient’s intraoperative anatomy could be matched and registered to the 3D model and allow for real-time 3D images and guidance of instruments (3,4). Although this method was shown to improve pedicle insertion accuracy as compared to traditional open methods, it has noted practical limitations (2,5). Intraoperative variations in patient positioning due to manipulation, breathing, or alteration of anatomy can affect the navigation accuracy and lead to misplaced screws (5). Additionally, the need to register each level of interest to try and compensate for potential disparities in the patient’s intraoperative positioning compared to the pre-operative CT adds substantial operative time to the procedure (5). Advances in technology now allow for the use of intraoperative imaging and frameless registration, which have been shown to both significantly decrease the time of the registration process, and also to improve screw placement accuracy as compared to the pre-operative CT navigation method (5). These improvements have made computer assisted navigation a standard, especially when dealing with complex anterior cervical spine cases.
It has already been shown that minimally invasive anterior transcorporeal foraminotomies and decompressions using intraoperative O-arm navigation are effective, efficient, and allow for the safe removal of diseased tissue while preserving the endplate, disc space, uncinate process, and neurovascular structures due to the anatomic detail offered in real time by navigation (1,6). This technology also may allow for smaller skin incisions and smaller drill holes than the conventional open approach for transcorporeal decompression (1). Additionally, anterior transpedicular pedicle screw placement using intraoperative navigation has been shown to have significantly higher rates of acceptable screw placement (66.7%) as compared to traditional fluoroscopy (42.6%) (7). In odontoid fractures requiring anterior screw placement, the use of intraoperative navigation has been shown to allow for optimal and safe screw placement (8). Intraoperative navigation also has the benefit of exposing the operating surgeon to less radiation than traditional fluoroscopy methods (9-11).
The authors would like to emphasize that utilization of navigation does not replace key knowledge of anatomy necessary to safely, and successfully perform complex spinal surgery. Navigation is to best used as a guide to support the surgeon, in performing the surgery. It should never be solely relied on without confirmation from anatomical landmarks as this can cause harm to the patient.
The purpose of this article is to provide a detailed overview of the stereotactic navigation process including the methods of setup and registration, and an overview of relevant clinical applications in regard to complex anterior cervical spine procedures. Previous report of this technique in anterior cervical spine surgery using fixed cranial-based referencing has not yet been described (6,12,13). We have already outlined our methods for navigation during complex posterior navigated surgery in a recent publication (14). Although there are many available navigation systems, our discussion will focus on the Medtronic O-arm and StealthStation (Medtronic, Minneapolis, MN, USA). This is solely based on the familiarity of the system by the senior authors, rather than any notion of clinical superiority. Some other commercially available systems include Iso-C C-arm (Siremobil Isco-C 3D; Siemens Medical Solutions, Erlangen, Germany) and NaviVision (VectorVision, BrainLab, Germany). Our discussion is meant to be generally applicable to all systems, and the spinal surgeon must ultimately decide on which system to use based on the pros and cons of each system, as well as his or her familiarity with the system. The described technique was found to be reproducible and effective, allowing cervical spine surgeons to perform more complex or minimally invasive procedures with safety and accuracy.
Method for employing stereotactic navigation
Room set up
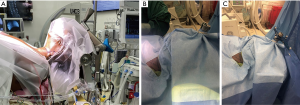
The authors’ preferred method of arranging the room is based upon what they believe to be the most fluid and ergonomically sound. Due to the space required to safely operate the O-arm, a large operative theater should ideally be utilized to avoid any sterility issues during the procedure. Upon entering the operating room, and after an introductory time out, the patient is sedated and intubated per anesthesia protocol. After intubation, neuromonitoring leads are placed and baseline motor functions are established. These are vital as they provide critical information to the surgeon about the baseline neurological status of the patient. The patient is then safely moved onto a regular operating room table in the supine position. The head is then placed into a Mayfield 360 head holder (Figure 1A). We recommend utilizing bacitracin on the pins prior to placement. Once the head is secured, the headboard is removed. It is imperative that the patient’s neck position be in a neutral appearing position. Avoid any excessive flexion or extension, as this could cause neurological injury. Prior to proceeding, we recommend obtaining a second motor function analysis. If there is concern for change, immediately readjust the patient’s head. The patient’s wrists are padded with foam and using the draw sheet, the patient’s arms are tucked at their side, ensuring that IV lines and electronic monitoring wires are free from kinks. After the surgeon chooses the side of the incision, the fluoroscopy attendant can begin moving the C-Arm in to the room, opposite of the incision. This will be utilized to confirm location of the corpectomy after exposure. The passive reference frame is then fixed on the Mayfield attachment, further described below. At the same time, the StealthStation can be placed at the head of the bed. This location is preferred to ensure that the reference frame and navigated instrument falls within the ideal navigated field of 0.95 and 2.4 meters. In an effort to preserve space, the O-Arm and the Navigation station should remain outside of the room while the C-Arm is in the room. Consequently, because of its short period of utilization in the operating room, if multiple navigation stations are available, many surgeons can utilize a single O-arm if their cases are slightly staggered.
Tracked instruments and the reference frame
The instruments utilized during the navigated portion of the procedure are all outfitted with a reflective sphere that will allow for optical tracking by an infrared camera. For our “standard” navigated instrument set, we utilize navigated drill and a ball-tip probe (chicken foot), although other instruments can be set up with navigation as needed. It is imperative for both the scrub technician and the surgeon to ensure that the spheres are completely seated on their mantle to ensure accurate tracking. Also, because the spheres rely on their ability to reflect the infrared signal, any disturbance to its glisten (such as blood, lap sponge, other tool) can prevent tracking of the sphere.
Prior to proceeding with frame attachment, the instruments and frame should be registered to the StealthStation. This is ideally performed by surgical scrub prior to incision. The frame and instrument registration merely confirm a spatial relationship between the two items, thus the frame need not be attached to patient to be registered. There is a risk of diminished accuracy if registration occurs while the frame is attached to the Mayfield as the likelihood of the frames being accidently moved is increased during the set up portion of the case.
Due to the paucity of space available during the anterior exposure of the spine, the authors prefer and recommend that the reference array is securely attached to the Mayfield (Figure 1B,C) within 6 to 8 inches of the operative field. This provides the surgeon with two main benefits. First, because it is fixed in space, it provides a stable reference position in relationship to the spine. Second, because it is located at the head of the patient, it is out of the surgical field. This allows for usage of a microscope and other larger instruments, should they be needed during the case. The non-sterile post is covered with clear plastic draping and secured with a sterile rubber band. Scissors are then used to cut a small window through the drape to allow insertion of the frame. The scissors are then discarded off the sterile field.
Approach and intraoperative spin
After the frame is attached, the Smith-Robinson surgical approach is performed. Once the deep retractors are at the level of the anterior longitudinal ligament, location of pathology is confirmed visually and confirmed using fluoroscopy. It is the authors preference to place an 18-gauge needle into the vertebral body rather than the disc space to avoid damaging an unrelated disc. Caspar pins are placed cranial and caudal to the intended discectomy and distraction is set. The authors prefer to perform an imaging spin at this point, following approach and Caspar pin distraction, as a spin prior to distraction may compromise navigation accuracy. One must be careful with regards to spinal cord compression and how much distraction is placed, as this may have the possibility of spinal cord insult. The wound is then irrigated with normal saline solution and left in place. This serves two purposes, first, it helps to prevent tissue desiccation, and secondly, it improves image quality by reducing air-tissue contrast. Although prolonged retraction of the soft tissues in the neck may lead to necrosis (15), this step is vital as any adjustments post-O-arm spin will render images inaccurate. This step will also save time post-spin, as the surgeon will be ready to proceed with discectomy immediately thereafter. Although there are commercially available O-arm sterile drapes, we utilize a method in which we use already present and abundant three-quarter surgical drapes. Using two three-quarter drapes, one from each side of the patient, the wound is covered, but the frame is left exposed. This is secured using towel clips, staples or other various clips. A sterile towel is then placed gently over the frame to protect it from the O-arm during O-arm closure. The C-Arm is removed from the room and the O-Arm is brought into the room and positioned accordingly. Prior to image acquisition, the green towel is removed leaving the frame exposed.
Direct referencing and localization
Due to the location of the frame, it can very easily be inadvertently moved by the surgeon or anesthesiologist during the process of removing the wound protective drape. To continually ensure accuracy and responsiveness of the instrumentation, it is our preference to verify accuracy by placing our probe on a bony landmark and ensure it correlates with the referenced images. Should the accuracy of the navigated system deteriorate, we may perform a re-registration of the system (re-spin of O-arm), begin to utilize fluoroscopy, or perform the procedure free-hand.
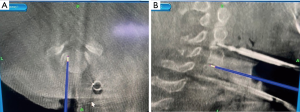
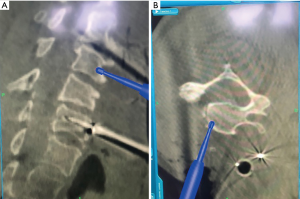
Once the spin is performed, microscope is brought into the operative field draped sterile. Microscope then remains in the field until the end of the case. A discectomy is performed at the cranial and caudal levels to aid in subsequent corpectomy (Figure 2A,B). Images of instruments and area of interest are displayed overhead on the operating room screens for the surgeon to monitor. On these screens, the authors prefer to only project the axial and sagittal images, although coronal images may also be useful. The same images along with other views (coronal) are located on the StealthStation monitor. Here, the operator of the O-arm is stationed. This operator can alter what image is projected on the surgeon screen, as well as trajectory projections of our instruments. To perform the corpectomy, a start point is selected. In this case, the mid-portion of the vertebral body is chosen as the start point using the burr (Figure 3A,B). The remainder of the surgical procedure can proceed as per surgeon preference.
Summary and case examples
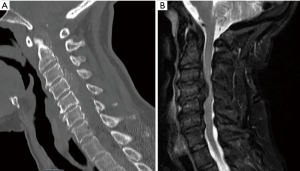
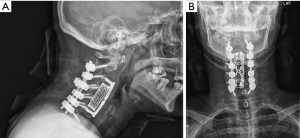
A 63-year-old patient with non-insulin dependent diabetes, and a twenty-pack year smoking history presented to our clinic with progressively worsening cervical myelopathy. Imaging showed severe stenosis from C3 to C5 with myelomalacia due to ossification of the posterior longitudinal ligament (Figure 4A,B). Given this, we performed a C4 and C5 full corpectomy and a C3 partial corpectomy, anterior fusion of C3 to C6, and a posterior spinal decompression and fusion of C2 to C7 (Figure 5A,B). Stereotactic navigation was used both anteriorly and posteriorly and allowed us to ensure compression anteriorly was completely resected.
Utilization of navigation in this specific case was helpful for many reasons. First, it allows for feedback during the discectomy portion of the case to ensure that we were sufficiently posterior and encountering the OPLL, and how much OPLL was present at the time of surgery. We left a thin shell of OPLL, as this was nearly incorporated into the dura. We were able to identify the exact extent of the ossification using navigation, to safely ensure the spinal cord was adequately decompressed. Secondarily, we are able to easily assess the medial-lateral extent of the ossification during the corpectomy using navigation. Lastly, by performing an additional O-Arm spin, prior to posterior stabilization, we could assess our anterior decompression while still in the operative theater. This provides an additional level of confidence ensuring the goal of the operation is completed prior to leaving the operating room.
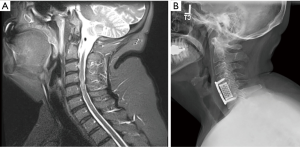
A 57-year-old patient with pulmonary hypertension, sleep apnea, and morbid obesity with a body mass index 58.9 presented to our clinic with severe progressively worsening cervical myelopathy. Imaging demonstrated cervical stenosis from C4−C6 with myelomalacia (Figure 6A). Also present was retropulsed intervertebral disc material. Given this, she underwent a complete C5 corpectomy (Figure 6B) with the aid of intra-operative stereotactic navigation. Given her large habitus navigation aided in this procedure to identify, correct levels as well as the medial and lateral extent of the corpectomy.
Limitations
Although intraoperative stereotactic navigation may not be necessary or cost-effective in performing routine procedures, in patients with complex anatomy, severe pathology, or other concerning features it can be an effective and reliable tool. It is important to not fall into over-confidence or complacency when using navigation assistance and repeated accuracy checks should be performed throughout the case to verify accuracy. Given the flexibility of the cervical spine, it takes relatively little movement to lose accuracy and this must be checked with anatomic landmarks or fluoroscopy as loss of accuracy of even several millimeters can have devastating complications. Further, additional operative time, learning curve, and interruptions to work flow are some disadvantages one may first encounter when utilizing this technique; however, as with any new technology, these disadvantages can be overcome with increased volume of cases and adaptability.
Conclusions
Navigation assisted spine surgery is a growing technology that can be of great aid in a variety of spine procedures. We present this technique guide in an effort to assist in increasing accuracy and efficiency in complex anterior cervical spinal surgery. The described technique was found to be reproducible and effective, allowing cervical spine surgeons to perform more complex or minimally invasive procedures with increased safety and accuracy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jss-20-580). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim JS, Eun SS, Prada N, et al. Modified transcorporeal anterior cervical microforaminotomy assisted by O-arm-based navigation: a technical case report. Eur Spine J 2011;20 Suppl 2:S147-52. [Crossref] [PubMed]
- Austin MS, Vaccaro AR, Brislin B, et al. Image-guided spine surgery: a cadaver study comparing conventional open laminoforaminotomy and two image-guided techniques for pedicle screw placement in posterolateral fusion and nonfusion models. Spine (Phila Pa 1976) 2002;27:2503-8. [Crossref] [PubMed]
- Schlenzka D, Laine T, Lund T. Computer-assisted spine surgery. Eur Spine J 2000;9 Suppl 1:S57-S64. [Crossref] [PubMed]
- Merloz P, Tonetti J, Pittet L, et al. Computer-assisted spine surgery. Comput Aided Surg 1998;3:297-305. [Crossref] [PubMed]
- Zhang W, Takigawa T, Wu Y, et al. Accuracy of pedicle screw insertion in posterior scoliosis surgery: a comparison between intraoperative navigation and preoperative navigation techniques. Eur Spine J 2017;26:1756-64. [Crossref] [PubMed]
- Quillo-Olvera J, Lin GX, Suen TK, et al. Anterior transcorporeal tunnel approach for cervical myelopathy guided by CT-based intraoperative spinal navigation: Technical note. J Clin Neurosci 2018;48:218-23. [Crossref] [PubMed]
- Patton AG, Morris RP, Kuo YF, et al. Accuracy of fluoroscopy versus computer-assisted navigation for the placement of anterior cervical pedicle screws. Spine (Phila Pa 1976) 2015;40:E404-10. [Crossref] [PubMed]
- Zou D, Zhang K, Ren Y, et al. Three-dimensional image navigation system-assisted anterior cervical screw fixation for treatment of acute odontoid fracture. Int J Clin Exp Med 2014;7:4332-6. [PubMed]
- Smith HE, Welsch MD, Sasso RC, et al. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J Spinal Cord Med 2008;31:532-7. [Crossref] [PubMed]
- Dusad T, Kundnani V, Dutta S, et al. Comparative Prospective Study Reporting Intraoperative Parameters, Pedicle Screw Perforation, and Radiation Exposure in Navigation-Guided versus Non-navigated Fluoroscopy-Assisted Minimal Invasive Transforaminal Lumbar Interbody Fusion. Asian Spine J 2018;12:309-16. [Crossref] [PubMed]
- Bratschitsch G, Leitner L, Stucklschweiger G, et al. Radiation Exposure of Patient and Operating Room Personnel by Fluoroscopy and Navigation during Spinal Surgery. Sci Rep 2019;9:17652. [Crossref] [PubMed]
- Jang SH, Cho JY, Choi WC, et al. Novel method for setting up 3D navigation system with skin-fixed dynamic reference frame in anterior cervical surgery. Comput Aided Surg 2015;20:24-8. [Crossref] [PubMed]
- Bredow J, Meyer C, Scheyerer MJ, et al. Accuracy of 3D fluoroscopy-navigated anterior transpedicular screw insertion in the cervical spine: an experimental study. Eur Spine J 2016;25:1683-9. [Crossref] [PubMed]
- Wallace N, Schaffer NE, Freedman BA, et al. Computer-assisted navigation in complex cervical spine surgery: tips and tricks. J Spine Surg 2020;6:136-44. [Crossref] [PubMed]
- Hu ZJ, Fang XQ, Zhou ZJ, et al. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am 2013;95:e192. [Crossref] [PubMed]


